This is an extraordinary time in urology. After decades of relative stagnation, patients with urothelial carcinoma are receiving approved immuno-oncologic drugs that significantly extend survival and are safer and more tolerable than chemotherapy. The success of these treatments in metastatic bladder cancer has generated strong interest and promising early results for their use in localized disease. With this shift comes exciting opportunities for urologists and associated care teams to hone their immuno-oncologic expertise and partner with medical and radiation oncologists and other physician-specialists to create innovative new models for high-quality cross-disciplinary care.
Metastatic Disease
The initial phases of oncologic drug development often start in late-stage disease, where patients have scant treatment options. Bladder cancer is no exception. As recently as 2016, there were no approved therapies for metastatic urothelial carcinoma that had progressed during or after platinum-based treatment. Only approximately 10% of these patients responded to second-line chemotherapy with single-agent paclitaxel, docetaxel, or vinflunine (in Europe), and median overall survival (OS) typically was only 6 to 7 months.1
Clinician-researchers tried and failed for decades to improve outcomes for these patients. Then, in October 2016, the international, phase III KEYNOTE-045 trial confirmed the superior efficacy and safety of the programmed cell death 1 (PD-1) inhibitor pembrolizumab (200 mg IV every 3 weeks for up to 2 years) versus investigator’s choice of chemotherapy with paclitaxel, docetaxel, or vinflunine.1 In the intention-to-treat population of 542 post-platinum patients with advanced transitional cell-predominant urothelial carcinoma, objective rates of response were 21.1% with pembrolizumab versus 11.4% with chemotherapy. Furthermore, after a median follow-up of 14.1 months, median duration of response was not reached with pembrolizumab versus 4.3 months with chemotherapy, and 68% of patients continued to respond to pembrolizumab for at least 12 months compared with only about 35% of chemotherapy recipients.
However, the most striking result from KEYNOTE-045 was overall survival (OS), a median of 10.3 months (95% CI, 8.0 to 11.8 months) in the pembrolizumab arm versus 7.4 months (6.1 to 8.3 months) with chemotherapy. Estimated rates of OS at 12 months were 43.9% and 30.7%, respectively, for a statistically significant hazard ratio (HR) for death of 0.73 (95% CI, 0.59 to 0.91; P = .0022).1
Pembrolizumab also was more tolerable than chemotherapy. Despite a median of 2 months more treatment exposure, only 61% of patients who received pembrolizumab developed treatment-related adverse events versus 90% of chemotherapy recipients.1 Pembrolizumab led to notably lower rates of grade 3 or higher toxicity (adverse events requiring intervention or changes in treatment) and serious adverse events.1
Pembrolizumab was the first agent to show an OS advantage over chemotherapy for the second-line treatment of metastatic urothelial carcinoma. This and its acceptable safety profile spurred its FDA approval for use in metastatic urothelial carcinoma that had progressed during or after platinum-based chemotherapy.2
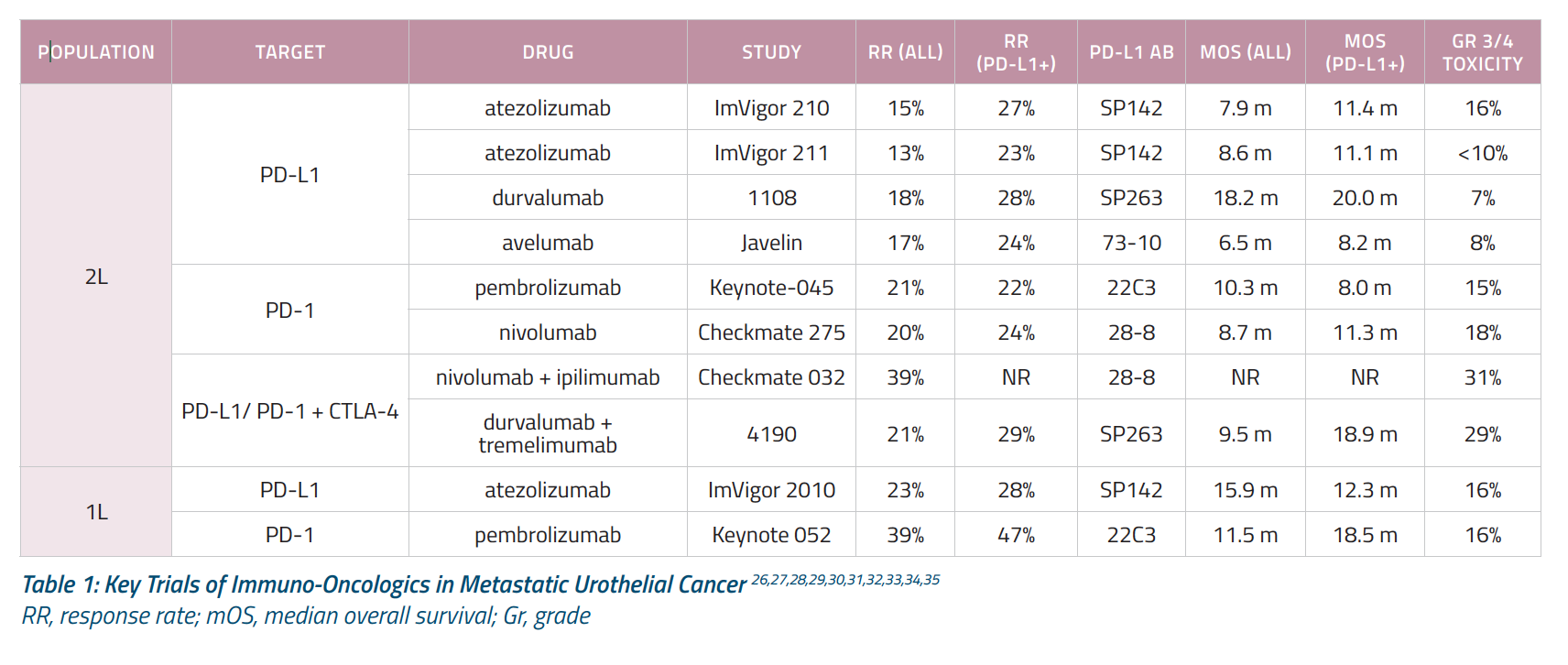
Table 1 summarizes results from KEYNOTE-045 as well as other key trials of immuno-oncologic agents in patients with metastatic urothelial cancer.1,3,4,5,6,7,8,9,10,11,12 A birds-eye view shows that rates of grade 3-4 toxicity are approximately 15% in the single-agent setting. Approximately 15% of unselected patients respond to second-line immuno-oncologic monotherapy and that this response increases to approximately 30% if we combine immuno-oncologics, such a PD-1 inhibitor and an anti-cytotoxic T-lymphocyte–associated antigen 4 (CTLA-4) agent. Used as first-line treatment, immuno-oncologic therapy induces responses in approximately 25% in unselected patients.
The results of these trials led to a flurry of approvals in the United States and Europe (Figure 1) that have revolutionized how we treat metastatic urothelial cancer. Since 2016, the FDA has approved five immune checkpoint inhibitors for use in post-platinum advanced or metastatic urothelial carcinoma. In addition to pembrolizumab (Keytruda), these include the PD-1 inhibitors atezolizumab (Tecentriq) and nivolumab (Opdivo) and the programmed death-ligand 1 (PD-L1) inhibitor durvalumab (Imfinzi), and avelumab (Bavencio).1,2,3,8,12 Additionally, atezolizumab and pembrolizumab have received FDA approval for use in platinum-ineligible patients as well as cisplatin-ineligible patients with high tumor levels of PD-L1 expression.6,7
Do Immuno-Oncologics Make Sense in Earlier-Stage Disease?
The efficacy of immuno-oncologic agents in advanced urothelial cancer has naturally raised questions about their potential use in earlier-stage disease. Could this approach achieve higher response rates and—most importantly—increase rates of cure for patients with muscle-invasive and non-muscle invasive bladder cancer?
To explore these questions, we first need to ask whether this approach makes sense biologically. Although urothelial cancer involves a host of potential targets for immunotherapy/immuno-oncology, I will focus on the PD-1/PD-L1 pathway because it is the target of currently approved agents in the metastatic setting. What role does this pathway play in localized disease?
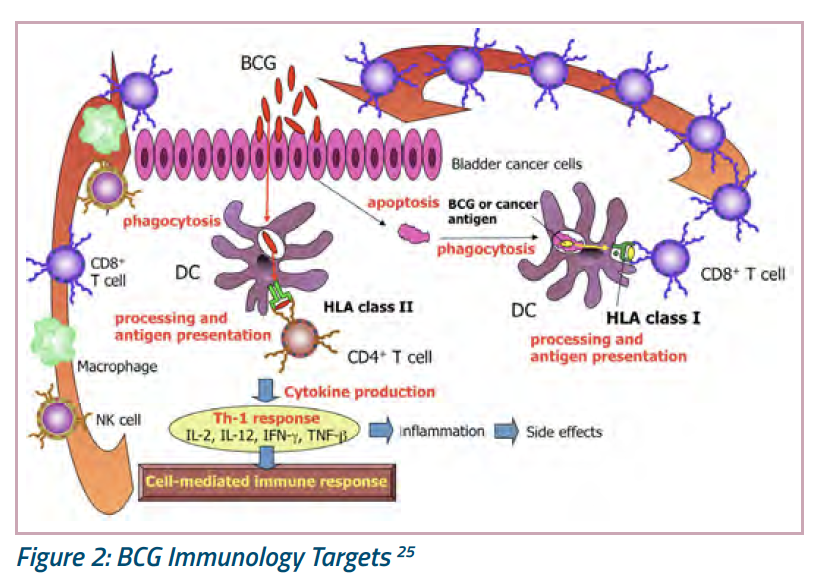
Hints come from recent progress in understanding the mechanism of action of intravesical Bacillus Calmette-Guérin (BCG). For years, we have known that BCG incites an inflammatory response, but recent advances in bench research tools have enabled us to take a closer look. These studies confirm that BCG affects both innate and adaptive immunity (Figure 2). With regard to the innate immune system, BCG molecules are phagocytosed, processed, and presented as antigens that trigger cell-mediated responses by natural killer (NK) cells and tumor-associated macrophages.24 However, BCG also can lyse urothelial cells (apoptosis), thereby releasing urothelial proteins that are phagocytosed and presented as antigens to surrounding immune cells that function in adaptive immunity (what we think of as the “memory” response). It remains unclear which type of immunity underlies the majority of BCG-induced cures, but most experts now agree that both innate and adaptive immunity are important to responses to intravesical BCG.
A second piece of evidence lies in data linking the PD-1 pathway to BCG resistance. In one study, Mayo Clinic investigators used immunohistochemical staining to examine pathologic specimens from 280 patients with high-risk non-muscle-invasive or muscle-invasive urothelial carcinoma.13 They found that stage progression correlated significantly with both high-grade tumor pathology and PD-L1 expression.13 Furthermore, in a subset of paired tumor specimens, the proportion with high PD-L1 expression rose from 19% in the BCG-naïve setting to 69% in the BCG-relapsed setting.13 This finding suggests that upregulation of the PD-1 pathway plays a role in BCG resistance, indicating that PD-1 blockers such as pembrolizumab might effectively treat these patients. Animal studies also provide useful preclinical data. In one study at the National Cancer Institute, researchers compared peritoneal (systemic) injections of either saline or the anti-PD-L1 antibody avelumab in mice with non-muscle-invasive bladder tumors.14 By day 21, avelumab produced superior tumor control based on both bladder weight and fluorescent imaging. Together, these studies support the role of immunity and the PD-1 pathway in both BCG treatment response and relapse. They justify the study of checkpoint inhibition in patients with localized urothelial cancer.
Key Ongoing Trials in Localized Disease
Several dozen trials of immuno-oncologic trials in localized bladder cancer are underway. They are focusing on adjuvant or neoadjuvant therapy for muscle-invasive disease as well as the use of immunotherapy for patients with BCG-unresponsive non-muscle-invasive disease. Furthermore, some trials are focusing on single-agent immunotherapy while others are exploring adding an immuno-oncologic to BCG or gemcitabine, with or without cisplatin or external beam radiation (and at least one trial [NCT02845323] is examining combination immuno-oncologic therapy: nivolumab with or without the anti-CD137 antibody urelumab).
This is a remarkable expansion of clinical trials in localized bladder cancer—in fact, a near-doubling of what we saw just a decade ago. We are witnessing intense drug development that I view as positive for patients and clinicians alike.
Muscle-Invasive Disease
For patients with muscle-invasive bladder cancer, three large randomized phase 3 registration trials are underway. In the adjuvant setting, ImVigor 010 (NCT02450331) is randomly assigning approximately 800 post-operative patients with urothelial cancer at high risk for recurrence to receive the PD-L1 inhibitor atezolizumab (1200 mg every 3 weeks for 1 year) or to undergo observation only, which is the current standard of care for this population post-cystectomy.
ImVigor 010 investigators have defined high-risk disease as pathologic T2-T4 or node-positive (N+) margin-negative disease in patients who have received prior cisplatin-based neoadjuvant chemotherapy, or T3-4 or N+ margin-negative disease in patients who are ineligible for or decline cisplatin and have not received neoadjuvant therapy. The primary endpoint of this trial is disease-free survival (DFS). Although trials of adjuvants for bladder cancer take longer to read out than those in the metastatic setting, topline results are expected within the next one to two years. If immuno-oncologic treatment with atezolizumab shows a statistically significant and clinically meaningful benefit after cystectomy, this could be practice-changing.
In a parallel vein, the phase 3 Alliance A031501 (AMBASSADOR) trial (NCT03244384) has randomly assigned approximately 740 post-operative patients with high-risk, muscle-invasive bladder or upper urinary tract urothelial cancer to undergo observation only or to receive pembrolizumab (200 mg once every 3 weeks for 1 year).17 Resembling ImVigor 010, high-risk disease is defined as pT2 or higher-grade or N+ disease in recipients of neoadjuvant chemotherapy or pT3 or higher-grade or N+ disease in those who refuse or are ineligible for cisplatin. This trial is being led in the United States by the Alliance for Clinical Trials in Oncology and in Europe by the European Organisation for Research and Treatment of Cancer (EORTC). Co-primary endpoints are DFS and OS.
Finally, the phase 3 CheckMate 274 trial (NCT02632409) is randomly assigning approximately 700 post-cystectomy patients with high-risk muscle-invasive bladder cancer to receive either nivolumab (3 mg/kg) or placebo every 2 weeks for 1 year.19 High-risk disease is defined similarly to the ImVigor 010 and AMBASSADOR trials. The primary endpoint is DFS.
Non-Muscle-Invasive Disease
High-risk non-muscle-invasive bladder cancer (NMIBC) includes any carcinoma in situ (CIS), T1 tumors, or large high-grade Ta tumors. Currently, radical cystectomy is recommended for high-risk NMIBC patients that are refractory to or have relapsed after BCG therapy,21 but perioperative risks and adverse implications for quality of life make this option infeasible or unacceptable for many of our patients. Consequently, high-risk NMIBC is an area of high unmet clinical need. For the past several years, the FDA has encouraged the use of single-arm clinical trials to hasten the development of effective and tolerable therapies for this population.22
Several such ongoing trials merit mention. First, the Southwest Oncology Group (SWOG) is leading a single-arm phase 2 study (NCT02844816) of atezolizumab (1200 mg every 3 weeks for up to 1 year) in BCG-unresponsive non-muscle-invasive bladder cancer.18 This trial has enrolled approximately 130 patients to date. Coprimary endpoints are 6-month complete response (CR) and relapse-free survival.
Similarly, the single-arm, open-label, phase 2 KEYNOTE-057 study (NCT02625961) is evaluating monotherapy with pembrolizumab (200 mg every 3 weeks for up to 2 years) in cystectomy-ineligible or cystectomy-refusing patients with high-risk BCG-unresponsive non-muscle-invasive bladder cancer, defined as high-grade Ta, T1, or CIS. Primary endpoints are CR for patients with CIS, and DFS for patients without CIS. Approximately 260 patients are planned to enroll.
Interim KEYNOTE-057 results were reported at the 2018 meeting of the European Society for Medical Oncology (ESMO).20 Among 103 heavily pretreated patients with CIS, pembrolizumab therapy produced 3-month complete response rates (CRs) in 38.8% (95% CI, 29.4% to 48.9%). Median time to CR was 12.4 weeks, and 72.5% of CRs persisted at last follow up with a median follow-up of 14 months. In all, 80% CRs lasted at least 6 months and 54% lasted at least 9 months. Among the 25% of patients who experienced recurrence, none progressed to muscle-invasive or metastatic disease during follow-up. These are encouraging results because they point to some clearance of tumor at 3 months. However, data are not yet mature enough to support conclusions about duration of response.
When evaluating whether to shift systemic treatment to earlier-stage disease, we must consider not only rates and types of toxicities, but also whether these particular side effects are acceptable to a wider community of patients and physicians. Thus far, KEYNOTE-057 findings indicate that pembrolizumab has a similar toxicity profile in earlier-stage as in advanced bladder cancer. In all, 12.6% of patients developed grade 3-5 treatment-related adverse events. Treatment-related adverse events that were immune-mediated were uncommon but included type 1 diabetes mellitus as well as adrenal insufficiency, hypophysitis, pruritus, and generalized rash (1 patient each, or 1%). Additionally, one patient died from complications of grade 3 treatment-emergent immune-mediated colitis that was inadequately managed with corticosteroid therapy. This unfortunate result reflects what we see in the metastatic setting, where approximately 0.5% of patients who receive immuno-oncologic monotherapy develop a potentially fatal autoimmune toxicity. These are rare events, but they underline the need to carefully monitor patients for toxicities and implement systems ahead of time to ensure that adverse events are detected quickly and managed appropriately.
Based on the interim results of KEYNOTE-057, investigators in December 2018 opened the phase 3 KEYNOTE-676 trial (NCT03519256), which is assessing combination therapy with pembrolizumab and BCG for patients with non-muscle-invasive bladder cancer that is persistent or recurrent after BCG induction. There are three additional registrational trials underway of systemically administered PD-1/PD-L1 agents in NMIBC populations. The four-arm, randomized phase 2 CheckMate 9UT (NCT03519256) study is comparing nivolumab (480 mg every 4 weeks), with or without the investigative agent BMS-986205 (100 mg per day), with or without BCG in patients with BCGunresponsive non-muscle-invasive bladder cancer.23 BMS-986205 is an immunologic modulator that targets indoleamine 2,3-dioxygenase 1 (IDO1) to promote the proliferation and activation of dendritic cells, NK cells, and T lymphocytes.22 Approximately 440 patients will be enrolled with primary endpoints of CR and RFS. In the BCG-naïve high-risk NMIBC population, the randomized phase 3 POTOMAC trial (NCT03528694) will enroll 975 patients to treatment with durvalumab (1500 mg every 4 weeks) with or without BCG with a disease-free survival (DFS) primary endpoint. Similarly, the randomized phase 3 ALBAN trial (NCT03799835) will enroll 614 patients to atezolizumab treatment (1200 mg every 3 weeks) with or without BCG also with a DFS primary endpoint. Collectively, the results of these studies should help answer questions about whether combining systemic and intravesical immunotherapies can improve the rate, depth, and duration of response in localized urothelial cancer.
Impact of Immuno-Oncologics on Urology Practice
Immuno-oncologic agents have so far produced durable responses advanced urothelial cancer and initial responses in high-risk localized BCG-unresponsive patients. We are not yet at the point of discussing their potential for cure, but we are seeing patients live longer than ever before. Translating these findings into real-world clinical practice requires careful planning and honest discussions with partners, care teams, and colleagues across disciplines. For urology practices, I recommend starting by exploring the group’s philosophy on establishing an advanced practice in this area. A single physician champion might develop immuno-oncologic expertise and see patients, but who will cover at 4:30 p.m. on a Friday, when that person is on vacation and a patient calls about a possible toxicity? We need clear communication with partners regarding expectations to ensure reliable coverage and overall success. Another early step is to establish dependable relationships with other specialties, such as medical and radiation oncology, pathology, and oncologic pharmacy. It is important that these are solid relationships to optimize the patient experience and outcomes.
On a related note, I suggest clarifying the infusion center infrastructure—will the urology practice provide this directly or refer patients to a hospital or outpatient center? Another consideration is how to keep stakeholders current on available immuno-oncologic drugs and regimens and best practices for their use. Both the urology and oncology fields will require significant education—most oncologists are unfamiliar with treating non-muscle-invasive bladder cancer and most urologists have not previously administered systemic immunotherapies. This is a good opportunity for educational advances in both disciplines. Finally, patients with high-risk muscle-invasive disease are at risk for metastasis, and we need a plan in place ahead of time to seamlessly transition patients to oncology or palliative care.
Summary
The use of immuno-oncologic agents leads to durable tumor responses in a minority of patients with metastatic urothelial carcinoma and is showing early promise for treating localized, high-risk BCG-nonresponsive or recurrent disease. Clinical trials are underway that should help clarify whether and how these drugs can be extended to earlier-stage settings, where the bar is higher with regard to both safety and successful clinical outcomes.15 As this research continues, we anticipate a better understanding of which patient subgroups will significantly benefit from immuno-oncologics and when and how to use combination regimens. The use of checkpoint inhibitors and other immuno-oncologic agents in earlier-stage patient populations will require urology group practices to hone their expertise, team education, cross-disciplinary relationships, and infrastructure for activities ranging from infusions to toxicity management to palliative care. This requires careful planning and communication but offers exciting chances to work across disciplines to significantly improve survival and quality of life for our patients. The shift of immuno-oncologic therapy to earlier-stage use is especially promising because this is where the possibility for cure is highest.
Written by: Noah M. Hahn, MD, was born in Valparaiso, Indiana and attended the University of Notre Dame where he graduated in 1994 with a degree in mechanical engineering. He finished his medical school training at the Indiana University School of Medicine in 2000. He completed a transitional year internship at Emory University in 2001, his internal medicine residency at Duke University in 2003, and his hematology and oncology fellowship at the Indiana University Simon Cancer Center (IUSCC) in 2006. Upon completion of his fellowship training, he was appointed to the IUSCC oncology faculty and served as the leader of their prostate and bladder cancer programs. In addition, he served as the chief scientific officer of the Hoosier Cancer Research Group (formerly the Hoosier Oncology Group) and the executive officer of the Big Ten Cancer Research Consortium. In April 2014, Dr. Hahn joined the Johns Hopkins University School of Medicine faculty as the director of the medical oncology bladder cancer program with the academic rank of associate professor of oncology and urology.
References
- Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017 Mar;376(11):1015-1026.
- U.S. Food & Drug Administration. Pembrolizumab (Keytruda): advanced or metastatic urothelial carcinoma. https://www.fda.gov/drugs/informationondrugs/approveddrugs/ucm559300.htm Last updated May 19, 2017. Accessed March 13, 2019.
- Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909-1920.
- Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol 2018 Jan;19(1):51-64.
- Sharma P, Callahan MK, Calvo E, et al. Efficacy and safety of nivolumab plus ipilimumab in metastatic urothelial carcinoma: first results from the Phase I/II CheckMate 032 study. Abstract presented at: 31st Annual Meeting and Associated Programs of the Society for Immunotherapy of Cancer (SITC 2016) MA, USA: 2016.
- Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet. 2017 Jan;389(10064):67-76.7.
- Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 2017 Nov;18(11):1483-1492.
- Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018 Feb 24;391(10122):748-757.
- Balar AV, Mahipal A, Grande E, et al. Abstract CT112: Durvalumab + tremelimumab in patients with metastatic urothelial cancer. AACR Meet Abstr Online. 2018 Jul;78(13):supplement.
- Vuky J, Balar AV, Castellano DE, et al. Updated efficacy and safety of KEYNOTE-052: A single-arm phase 2 study investigating first-line pembrolizumab (pembro) in cisplatin-ineligible advanced urothelial cancer (UC). J Clin Oncol 2018;36(15_suppl):4524-4524.
- Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: Updated results from a phase 1/2 open-label study. JAMA Oncol 2017;3(9):e172411.
- Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. T Lancet Oncol 2017 Mar;18(3):312-322.
- Inman BA, Sebo TJ, Frigola X, et al. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007 Apr;109(8):1499-505.
- Vandeveer AJ, Fallon JK, Tighe R, Sabzevari H, Schlom J, Greiner JW. Systemic Immunotherapy of Non-Muscle Invasive Mouse Bladder Cancer with Avelumab, an Anti-PD-L1 Immune Checkpoint Inhibitor. Cancer Immunol Res. 2016 May;4(5):452-462.
- Hahn NM, Necchi A, Loriot Y, et al. Role of checkpoint inhibition in localized bladder cancer. Eur Urol Oncol. 2018 Aug;1(3):190-198.
- U.S. Food and Drug Administration. BCG-Unresponsive Nonmuscle Invasive Bladder Cancer: Developing Drugs and Biologics for Treatment Guidance for Industry. https://www.fda.gov/ downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM529600.pdf Accessed March 14, 2019.
- Apolo AB, Rosenberg JE, Kim WY, et al. Alliance A031501: Phase III randomized adjuvant study of MK-3475 (pembrolizumab) in muscle-invasive and locally advanced urothelial carcinoma (MIBC) (AMBASSADOR) versus observation. J Clin Oncol. 2019 Feb;37(7_suppl).
- Singh P, Tangen C, Lerner SP, et al. S1605: Phase II trial of atezolizumab in BCG-unresponsive non-muscle invasive bladder cancer. J Clin Oncol 2017 May;35(15_suppl).
- Bajorin D, Galsky MD, Gschwend JE, et al. A Phase III, randomized, double-blind, multicenter study of adjuvant nivolumab vs placebo in patients (pts) with high-risk invasive urothelial carcinoma (UC; CheckMate 274). Ann Oncol 2017 Sep;28(25_suppl).
- De Wit R, Kulkarni GS, Uchio EM, et al. Pembrolizumab for high-risk non-muscle invasive bladder cancer unresponsive to BCG: phase 2 Keynote 057 trial. Abstract presented at: ESMO 2018 Congress. https://oncologypro.esmo.org/Meeting-Resources/ESMO-2018-Congress/ Pembrolizumab-for-High-Risk-HR-Non-Muscle-Invasive-Bladder-Cancer-NMIBC-Unresponsiveto-Bacillus-Calmette-Guerin-BCG-Phase-2-KEYNOTE-057-Trial Accessed March 14, 2019.
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Bladder Cancer. Version 1.2019—December 20, 2018. https://www.nccn.org/professionals/physician_gls/PDF/bladder.pdf Accessed March 14, 2019.
- National Cancer Institute. NCI Drug Dictionary: IDO1 inhibitor BMS-986205. https://www.cancer.gov/publications/dictionaries/cancer-drug/def/ido1-inhibitor-bms-986205 Accessed March 16, 2019.
- Hahn NM, Chang SS, Meng M, et al. A phase II, randomized study of nivolumab (nivo) or nivo plus BMS-986205 with or without intravesical Bacillus Calmette-Guerin (BCG) in BCG-unresponsive, high-risk, non-muscle invasive bladder cancer (NMIBC): CheckMate 9UT. J Clin Oncol. 2019 Feb;37(7_suppl).
- Kitamura H, Tsukaoto T. Immunotherapy for urothelial carcinoma. Current status and perspectives. Cancers 2011;3(3):3055-3072.
- Kitamura, H.; Tsukamoto, T. Immunotherapy for Urothelial Carcinoma: Current Status and Perspectives. Cancers 2011, 3, 3055-3072
- Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet. 2016;387(10031):1909-1920.
- Patel MR, Ellerton J, Infante JR, et al. Avelumab in metastatic urothelial carcinoma after platinum failure (JAVELIN Solid Tumor): pooled results from two expansion cohorts of an open-label, phase 1 trial. Lancet Oncol 2018 Jan;19(1):51-64.
- Sharma P, Callahan MK, Calvo E, et al. Efficacy and safety of nivolumab plus ipilimumab in metastatic urothelial carcinoma: first results from the Phase I/II CheckMate 032 study. Abstract presented at: 31st Annual Meeting and Associated Programs of the Society for Immunotherapy of Cancer (SITC 2016) MA, USA: 2016.
- Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol 2017 Sep 26. [Epub ahead of print]
- Powles T, Durán I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): a multicentre, open-label, phase 3 randomised controlled trial. Lancet. 2018 Feb 24;391(10122):748-757.
- Bellmunt J, de Wit R, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med 2017 Mar;376(11):1015-1026.
- Balar AV, Mahipal A, Grande E, et al. Abstract CT112: Durvalumab + tremelimumab in patients with metastatic urothelial cancer. AACR Meet Abstr Online. 2018 Jul;78(13):supplement.
- Vuky J, Balar AV, Castellano DE, et al. Updated efficacy and safety of KEYNOTE-052: A single-arm phase 2 study investigating first-line pembrolizumab (pembro) in cisplatin-ineligible advanced urothelial cancer (UC). J Clin Oncol;36(15_suppl):4524-4524.
- Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: Updated results from a phase 1/2 open-label study. JAMA Oncol 2017;3(9):e172411.
- Sharma P, Retz M, Siefker-Radtke A, et al. Nivolumab in metastatic urothelial carcinoma after platinum therapy (CheckMate 275): a multicentre, single-arm, phase 2 trial. T Lancet Oncol 2017 Mar;18(3):312-322.


