Barcelona, Spain (UroToday.com) Durvalumab is a PD-L1 inhibitor with efficacy in platinum-refractory advanced urothelial cancer and approved by the FDA after a reported response rate of 18%.1 FGFR targeted therapy (ie. erdafitinib) also has FDA approval in biomarker-selected patients with urothelial cancer based on a response rate of 40%.2 At the ESMO 2019 non-prostate cancer session, Professor Tom Powles presented the first results of BISCAY, a combination immune therapy trial investigating targeted therapy inhibitors (FGFR1,2,3, PARP, TORC 1 + 2) with a PD-L1 monotherapy arm (durvalumab alone) as a non-randomized control.
As follows is an overview of targeted therapies combined with durvalumab: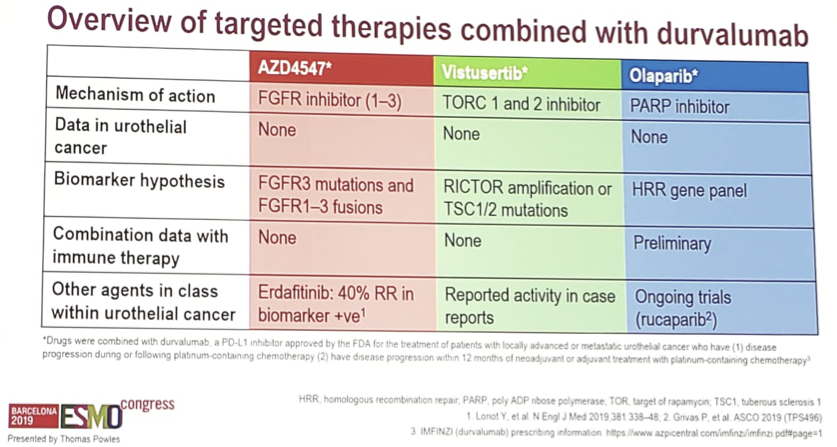
For BISCAY, platinum refractory, immuno-therapy naïve urothelial carcinoma patients were allocated, depending on tumour DNA alterations determined by next generation sequencing, to the following arms:
- Arm A – randomization of durvalumab + the FGFR inhibitor AZD4547 vs AZD4547 monotherapy for FGFR mutations/fusions
- Arm B – durvalumab + the PARP inhibitor olaparib for BRCA1/2, ATM and HRR gene alterations and unselected patients
- Arm E – durvalumab + the mTOR inhibitor vistusertib for enriched TSC1/2 and RICTOR gene alterations
- Arm D – durvalumab only

The primary objectives included safety and tolerability. Key secondary objectives included assessment of ORR and OS rate. Efficacy analysis was explored based on PD-L1 expression and tumour mutational burden. The investigators set an ORR of >60% as a meaningful response rate.
As of March 2019, of 391 patients whose tumours were screened with next generation sequencing, 108 started the study drug and had a baseline tumor assessment. Confirmed responses were assessed according to RECIST 1.1 and ranged from 20.0% to 35.7%. Tumor mutational burden and PD-L1 were inconsistent across arms: for example, 43% tumor mutational burden for Arm E vs 5% in Arm A combination and 18% in Arm D.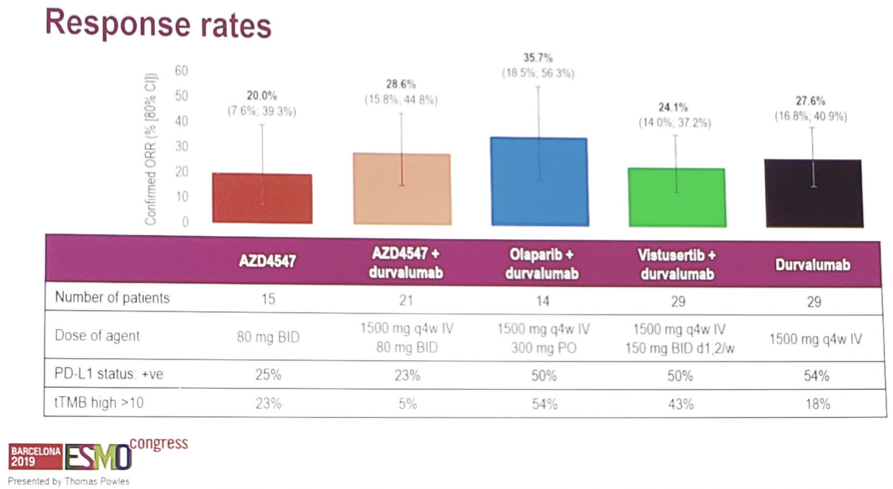
Durvalumab monotherapy (n = 29) had an ORR of 28% (80% CI 17% - 41%) with 12% (80% CI 3.2% - 28%) of patients alive and progression-free, and 45% (80% CI 24% - 63%) of patients alive at 12 months. Complete responses were not prominent in any study arm. Adverse events leading to treatment discontinuation ranged from 7.1%-26.7%, and adverse events leading to withdrawal, interruption, or dose reduction ranged from 10.3%-86.7%.
Dr. Powles concluded his presentation of BISCAY with several take-home messages:
- This trial employed a biomarker-driven, innovative trial design to maximize efficiency
- The combinations investigated were tolerable and adverse events were in line with historical controls
- AZD4547 monotherapy had activity similar to AZD4547 plus durvalumab combination and PD-L1 expression was lower in the FGFR arm than in other arms
- High tumor mutational burden was more prevalent in the olaparib arm and response rate was in line with other arms
- Vistusertib plus durvalumab had response rates and PFS in line with durvalumab alone
- No combination treatments and biomarker selection reached the prespecified efficacy target
- This data raises stimulating questions about potential future treatment strategies for metastatic urothelial carcinoma
NCT02546661
Presented by: Thomas Powles, MBBS, MRCP, MD, Professor of Genitourinary Oncology, Lead for Solid Tumour Research at Barts Cancer Institute, Director of Barts Cancer Institute, London UK.
Co-authors: A. Balar,2 G. Gravis,3 R. Jones,4 A. Ravaud,5 J. Florence,6 P. Grivas,7 D. Petrylak,8 M. Galsky,9 J. Carles,10 S. Sridhar,11 H.-T. Arkenau,12 D. Carroll,13 J. Decesare,14 F. Mercier,14 D. Hodgson,15 J. Stone,16 J. Cosaert,17 D. Landers18
2. NYU Langone, New York, US
3. Institute Paoli Calmettes, Marseille, FR
4. University of Glasgow, Glasgow, UK
5. CHU Bordeaux Hopital St. André, Bordeaux, FR
6. Centre Francois Balesse, CAEN, FR
7. University of Washington Seattle Cancer Care Alliance, Seattle, US
8. Yale Cancer Center, New Haven, US
9. Icahn School of Medicine at Mount Sinai Hospital, New York, US
10. Vall d'Hebron University Hospital, Barcelona, ES
11. Princess Margaret Hospital, Toronto, CA
12. Sarah Cannon Research Institute UK, London, UK
13. AstraZeneca, Cambridge, UK
14. AstaZeneca, Melbourne, AU
15. AstraZeneca, Boston, US
16. AstraZeneca Academy House, Cambridge, UK
17. AstraZeneca UK
18. Astrazeneca, Macclesfield, UK
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 2019 European Society for Medical Oncology annual meeting, ESMO 2019 #ESMO19, 27 Sept - 1 Oct 2019 in Barcelona, Spain
References:
1. Powles T, O’Donnell PH, Massard C, et al. Efficacy and safety of durvalumab in locally advanced or metastatic urothelial carcinoma: Updated results from a phase 1/2 open-label study. JAMA Oncol 2017;3(9):e172411.
2. Loriot Y, Necchi A, Park SH, et al. Erdafitinib in Locally Advanced or Metastatic Urothelial Carcinoma. N Engl J Med 2019 Jul 25;381(4):338-348.


