
This abstract provides data on 43 patients with HER2+ mUC who have been treated with 1 or more prior systemic agents. Key inclusion criteria are shown below:

86% of these patients had visceral metastases and 33% of patients had two prior lines of treatment. 19% of patients had prior immune checkpoint inhibitor therapy. All patients were HER2 positive as defined by IHC 2+ or 3+. A summary of baseline characteristics is shown below.
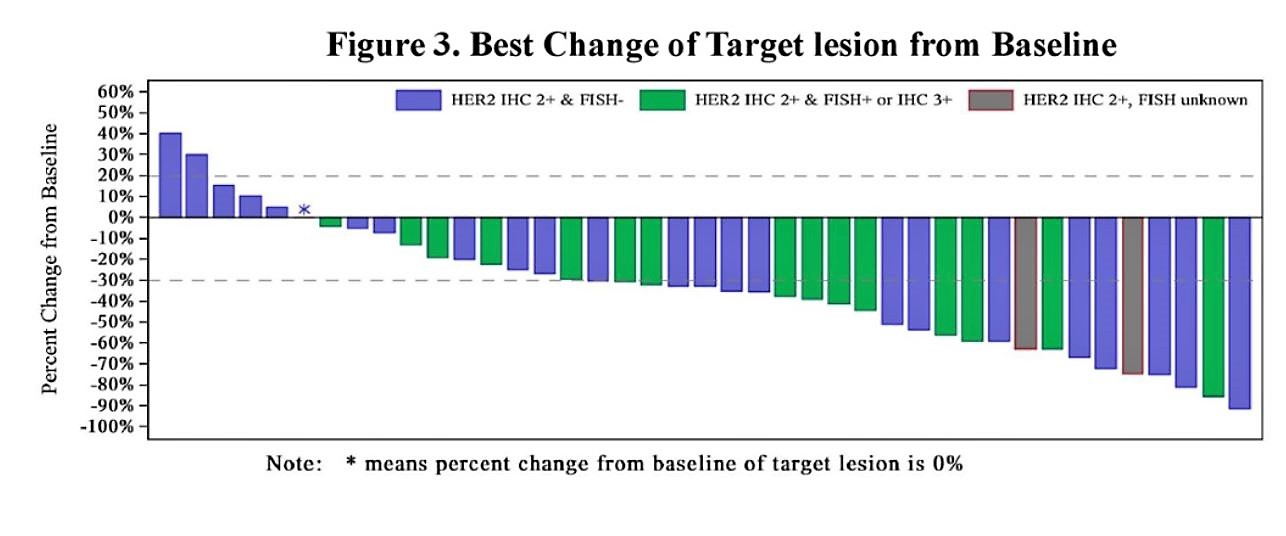
The objective response rate (ORR) was 60.5% for all patients with a disease control rate of 90.7%. For patients with liver metastases, the objective response rate was 70% (14/20). Confirmed ORR was 51.2%. As you can see from the graph below, there are several patients with ongoing responses past 30 weeks.

All patients who were classified as IHC2+ and FISH+ or IHC3+ had shrinkage of the tumor.
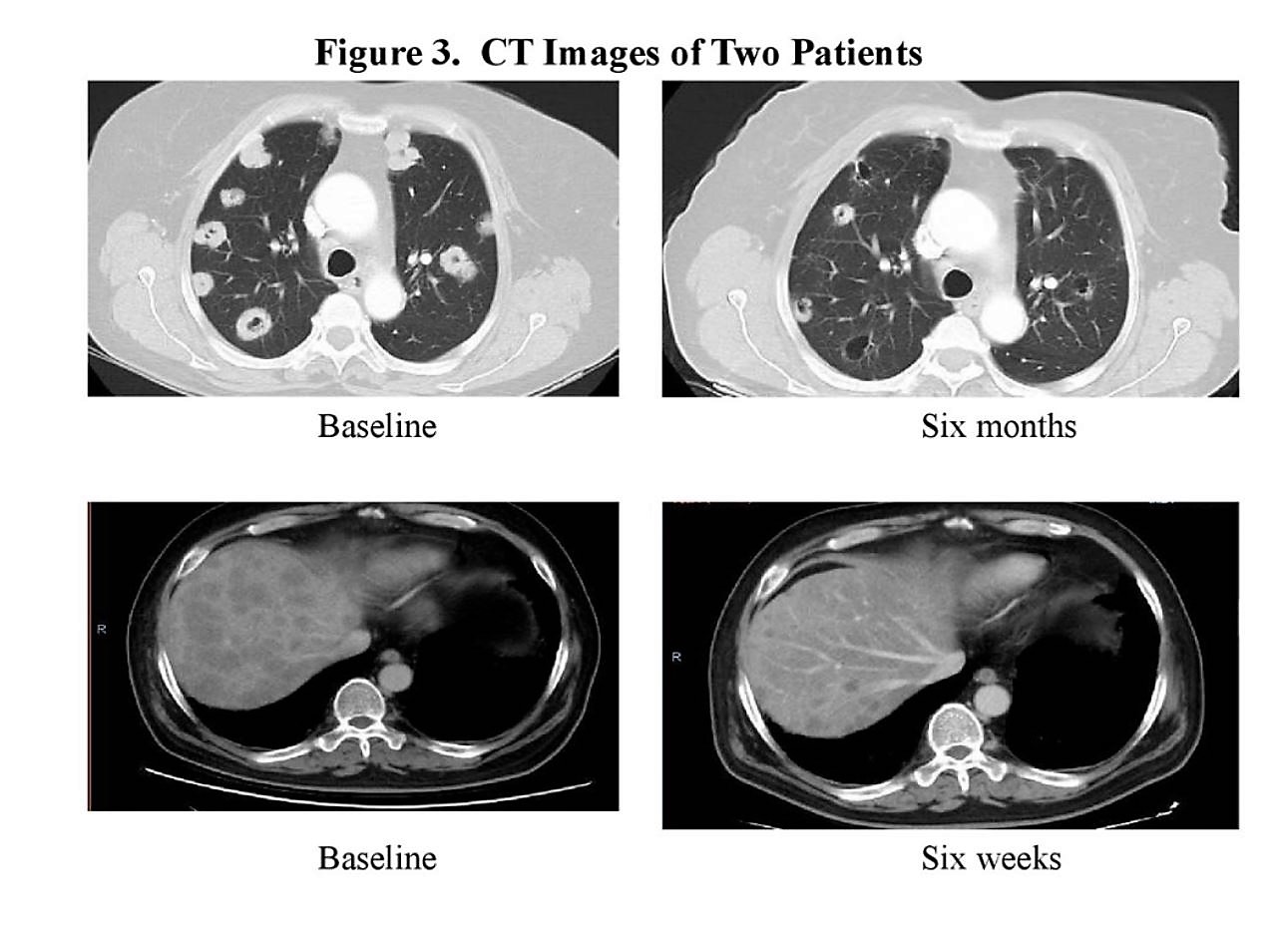
There were impressive radiographic examples of response as shown below.
The most common treatment-related adverse events (AEs) were leukopenia (51.2%), hypoesthesia (41.9%), alopecia (41.9%), neutropenia (37.2%), fatigue (34.9%), ALT increase (32.6%), and AST increase (32.6%). One AE of note was complete and partial small bowel obstruction which occurred in 4.7% of patients.
This abstract provides promising data for a HER2+ ADC which was able to achieve a 60.5% ORR. Overall progression-free survival (PFS) and overall survival (OS) data is not yet mature but the median PFS for the first 9 patients on the study was 7.8 months. The prevalence HER2 overexpression in UC widely varies depending on the study, and interestingly appears to vary depending on geography. In one study, HER2 alterations were seen in 27% of patients from Spain, but only 4% in patients from Greece.4 HER2 is an important biomarker for patients with mUC and the data from this study certainly warrants further evaluation in larger cohorts of HER2+ mUC. A larger study is underway to validate these results and continue to evaluate safety.
Presented by: Xinan Sheng, MD, Peking University Cancer Hospital, Beijing, China
Written by: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, @TheRealJasonZhu, at the 2019 ASCO Annual Meeting #ASCO19, May 31- June 4, 2019, Chicago, IL USA
References:
- Alley SC, Okeley NM, Senter PD. Antibody-drug conjugates: targeted drug delivery for cancer. Current opinion in chemical biology 2010;14:529-37.
- Rosenberg JE, Sridhar SS, Zhang J, et al. Updated results from the enfortumab vedotin phase 1 (EV-101) study in patients with metastatic urothelial cancer (mUC). American Society of Clinical Oncology; 2018.
- Gong J, Shen L, Wang W, Fang J. Safety, pharmacokinetics and efficacy of RC48-ADC in a phase I study in patients with HER2-overexpression advanced solid cancer. American Society of Clinical Oncology; 2018.
- Bellmunt J, Werner L, Bamias A, et al. HER2 as a target in invasive urothelial carcinoma. Cancer medicine 2015;4:844-52.


