(UroToday.com) On the second day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022 focused on urothelial carcinoma, in Poster Session B, Dr. Galsky presented an analysis of the CheckMate274 trial of adjuvant nivolumab in urothelial carcinoma. In the primary publication of the trial, adjuvant nivolumab improved disease-free survival (DFS) with both in the intent-to-treat population (hazard ratio [HR], 0.70; 98.22% confidence interval [CI], 0.55–0.90; P < 0.001) and among patients (pts) with tumor programmed death ligand 1 (PD-L1) expression ≥ 1% assessed by the tumor proportion score (TPS) (HR, 0.55; 98.72% CI, 0.35–0.85; P < 0.001). However, an exploratory subgroup analysis showed a trend toward a DFS benefit with nivolumab in patients with TPS < 1% (0.82; 95% CI, 0.63–1.06).
Thus, to better understand the relationship between PD-L1 expression and the efficacy of nivolumab in this space, the authors undertook an analysis of DFS based on PD-L1 expression in both tumor and immune cells using the combined positive score (CPS).
While previously published, to briefly summarize, CheckMate 274 is a phase 3, randomized, double-blind, multicenter randomized controlled trial comparing nivolumab (240 mg IV every two weeks for 1 year) versus placebo in the adjuvant setting following radical surgery for patients with high-risk muscle-invasive urothelial carcinoma.

The primary endpoints of the study are DFS in the intent-to-treat population and among with TPS ≥ 1%. TPS was assessed using the Dako PD-L1 IHC 28-8 pharmDx and CPS was determined retrospectively from previously stained immunohistochemistry slides using the CPS algorithm. CPS was calculated as the number of both PD-L1 positive tumor and immune cells divided by the number of viable tumor cells in the evaluable tumor area, multiplied by 100. Additionally, TPS was similarly calculated with the number of PD-L1 positive tumor cells as the numerator. This analysis only included those patients who had both quantifiable CPS and TPS.
Among 629 patients with quantifiable TPS and CPS, 249 (40%) had TPS ≥ 1% (of whom 124 were randomized to nivolumab and 125 to placebo), 380 (60%) had TPS < 1% (of whom 191 were randomized to nivolumab and 189 to placebo), 557 (89%) had CPS ≥ 1 (of whom 281 were randomized to nivolumab and 276 to placebo), and 72 (11%) had CPS < 1 (of whom 34 were randomized to nivolumab and 38 to placebo).
Among patients with TPS <1%, 81% (n = 309) had CPS ≥ 1. In this subset of patients, the median DFS (95% CI) was significantly longer for those patients who received nivolumab (median 19.2 months, 95% CI 15.6–33.4) compared to placebo (median 10.1 months, 95% CI 8.2–19.4) (hazard ratio 0.73, 95% CI, 0.54–0.99).
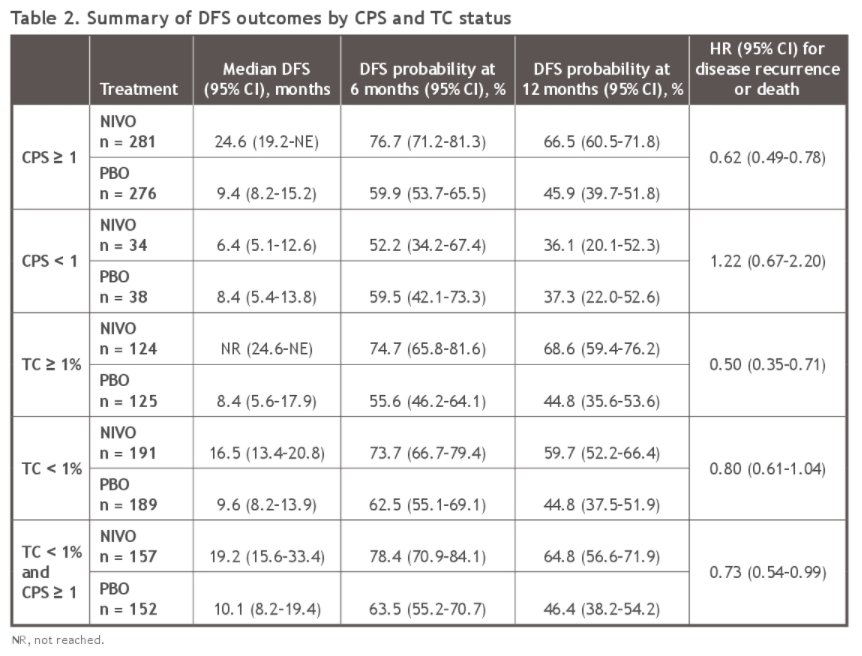
This exploratory subgroup analysis of CheckMate274 by PD-L1 expression shows a higher proportion of patients with CPS≥ 1 than TPS ≥ 1%, with most patients with TPS < 1% having a CPS ≥ 1. Thus, with the observed benefit of nivolumab in the CPS ≥ 1 subgroup, this may explain the observed benefit of adjuvant nivolumab in patients with TPS < 1%.
Presented by: Matt D. Galsky MD, FASCO, Icahn School of Medicine at Mount Sinai, New York, NY

