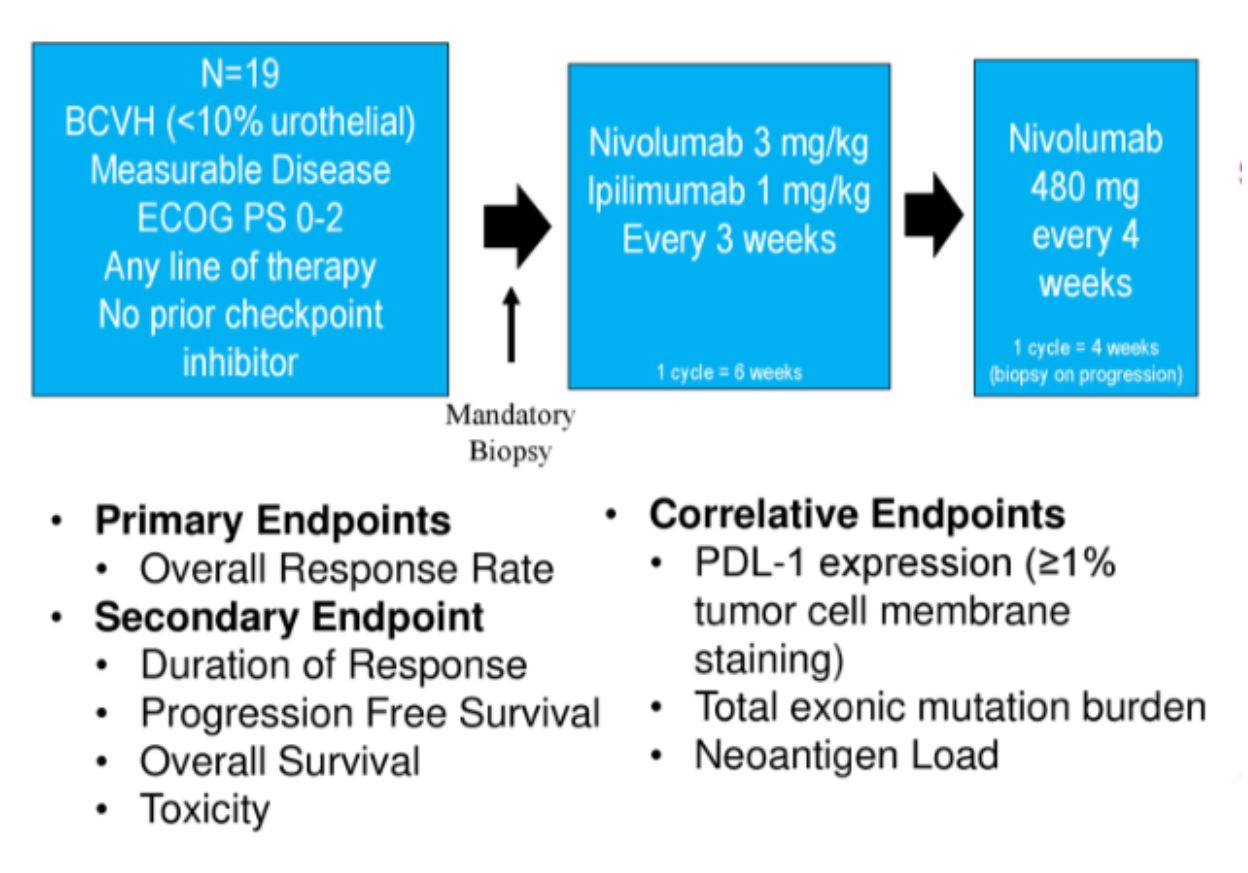
This abstract provides data on 19 patients with BCVH. Patient characteristics are below.

The variant histologies which were represented included squamous cell carcinoma (n=6), small cell carcinoma (n=3), adenocarcinoma (n=3), urachal (n=5), plasmacytoid (n=1), and spindle cell carcinoma (n=1).

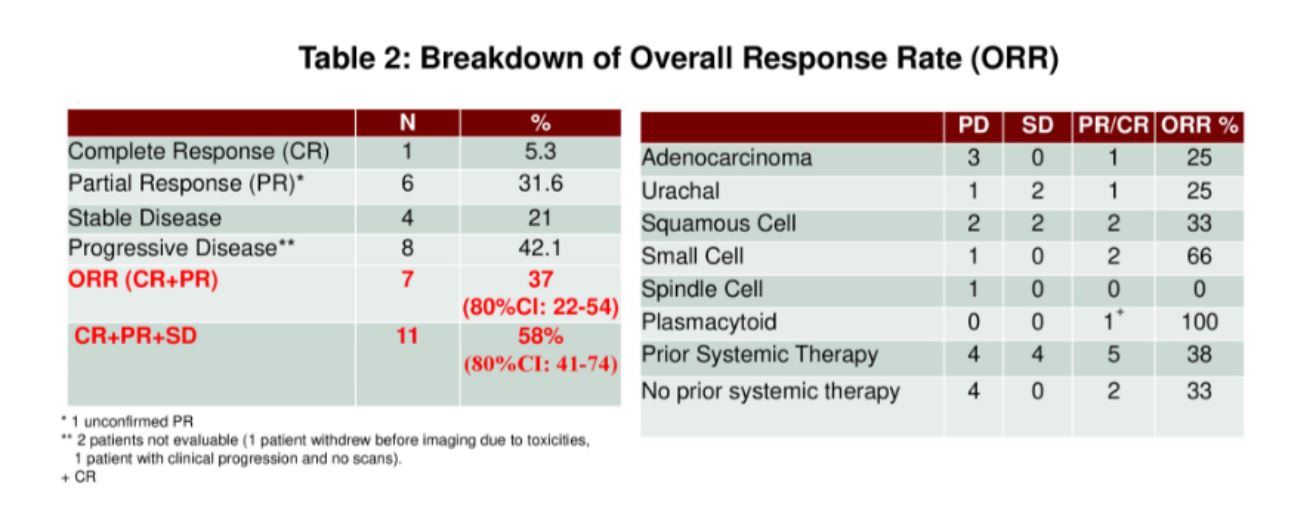
After a median follow-up of 3.6 months, the objective response rate was 37% (small cell – 2, urachal – 1, and plasmacytoid – 1).
In terms of safety, 16% (3/19) patients developed grade 3 treatment-related toxicity and 1 patient had grade 4 toxicity. Fatigue, rash, and hypothyroidism were the most common adverse events (AEs) overall.

Urothelial bladder cancer represents >90% of all bladder cancer diagnoses in the United States and a number of immunotherapy trials have changed the way we manage urothelial carcinoma over the past few years. However, for patients with non-urothelial bladder cancer, much is unknown regarding the efficacy of immunotherapy. One prior case report described a patient with squamous cell carcinoma who had a complete response to pembrolizumab.2 This abstract demonstrates that the combination of ipi/nivo may be an effective treatment for patients with bladder cancer of variant histologies.
Presented by: Bradley Alexander McGregor, MD, Chair, Lank Center for Genitourinary Oncology, Dana-Farber Cancer Institute, Boston, Massachusetts
Written by: Jason Zhu, MD. Fellow, Division of Hematology and Oncology, Duke University, @TheRealJasonZhu, at the 2019 ASCO Annual Meeting #ASCO19, May 31- June 4, 2019, Chicago, IL USA
References:
- Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. New England Journal of Medicine 2018;378:1277-90.
- Kao C, McNamara M, Alley C, et al. A Complete Response After Pseudo-progression: Pembrolizumab for Metastatic Squamous Cell Carcinoma (SCC) of the Bladder. Clinical genitourinary cancer 2019.


