- After platinum-containing chemotherapy, or
- Considered cisplatin ineligible and PD-L1 positive, or
- Ineligible for any platinum therapy, irrespective of PD-L1 status (US only)
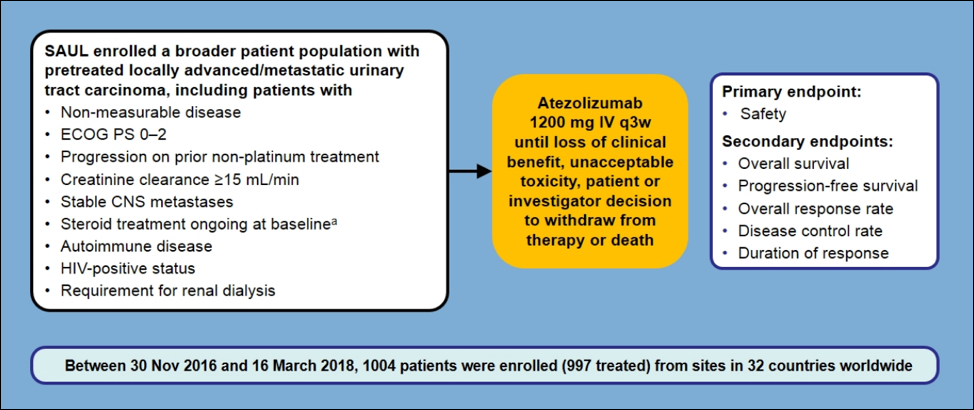
The median patient age was 68 (range 34-93), 77% were male, 47% of patients were ECOG 1 and 10% ECOG 2, 38% of patients had no prior treatment, 54% had 1 prior treatment, and 28% were PD-L1 positive. The majority of tumors were urothelial carcinoma (95%), 75% were in the bladder, 12% in the renal pelvis and 10% in the ureter.
The adverse event profile was consistent with previous IO trials. The rate of any treatment-related adverse events was 53%; the most common specific adverse events were asthenia and fatigue. In the ITT population (n=1,004), the median overall survival (OS) was 8.7 months (95%CI 7.8-9.9), the 6-month OS rate was 60% (95%CI 57-63%), and the 12-month OS rate was 41% (95%CI 38-44%). The median PFS in the ITT population was 2.2 months (95%CI 2.1-2.4), 6-month PFS rate was 29% (95%CI 26-32%), and the 12-month PFS rate was 17% (95%CI 15-20%). The objective response rate was 13% (95%CI 11-16%), with 3% of patients having a complete response, 11% partial response, 26% stable disease, and a disease control rate of 40%. There were 6% of patients that received treatment and discontinued secondary to an adverse event.
In a subgroup analysis assessing patients that would have been candidates for the phase 3 randomized controlled trial IMvigor211 (n=643)3, the median OS was 10.0 months (95%CI 8.8-11.9) and the 12-month OS rate was 46% (95%CI 41-50%). The median PFS was 2.3 months (95%CI 2.2-2.6), and the objective response rate was 14% (95%CI 11-17%). Similarly, when comparing SAUL to other criteria/trials, Dr. Merseburger notes that these OS curves are nearly identical:
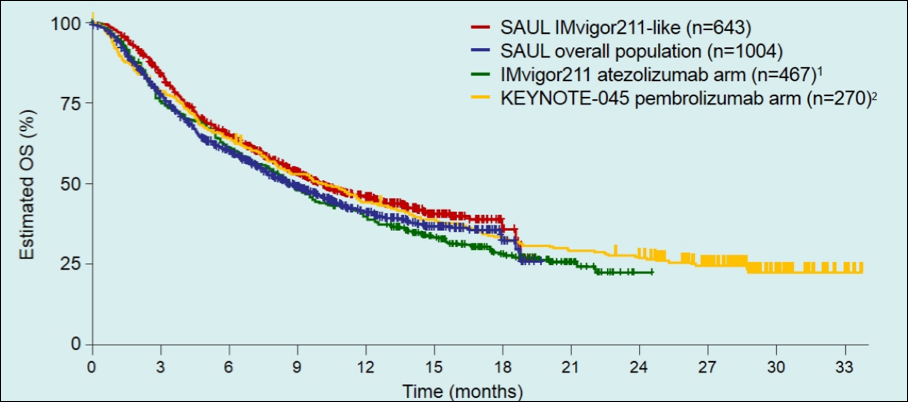
Dr. Merseburger concluded his presentation of the preliminary results of SAUL with several take-home messages:
- SAUL is the largest prospective clinical trial of immunotherapy in advanced urinary tract carcinoma
- Atezolizumab is a tolerable and effective treatment, even in complex comorbid populations
- The efficacy overall and in the IMvigor211-like subgroup is consistent with previous pivotal anti-PD-L1/PD-1 urothelial carcinoma trials: the median OS in SAUL was 10.0 months in the IMvigor211-like population (n=643) and 8.7 months in the ITT population (n=1,004)
- These results support the use of atezolizumab in urinary tract carcinoma, including in patients with limited treatment options
Presented by: Axel S. Merseburger, MD, Chairman of the Department of Urology, University Hospital Schleswig-Holstein, Lübeck, Germany
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University - Medical College of Georgia, Twitter: @zklaassen_md at the 34th European Association of Urology (EAU 2019) #EAU19 conference in Barcelona, Spain, March 15-19, 2019.
References:
- Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016;387(10031):1909-1920.
- Balar AV, Galsky MD, Rosenberg JE, et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: A single-arm, multicentre, phase 2 trial. Lancet 2017;389(10064):67-76.
- Powles T, Duran I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomized controlled trial. Lancet 2018;391:748-757.


