Advanced Prostate Cancer
- Written by: Zachary Klaassen, MD, MSc Associate Professor of Urology Urologic Oncologist Medical College of Georgia, Georgia Cancer Center Augusta, GA & Rashid Sayyid, MD, MSc Urologic Oncology Fellow University of Toronto Toronto, Ontario, Canada
- References:
- FDA Approves Olaparib with Abiraterone and Prednisone (Or Prednisolone) for BRCA-Mutated Metastatic Castration-Resistant Prostate Cancer.
- FDA Approves Niraparib and Abiraterone Acetate plus Prednisone for BRCA-Mutated Metastatic Castration-Resistant Prostate Cancer.
- FDA Approves Talazoparib with Enzalutamide for HRR Gene-Mutated Metastatic Castration-Resistant Prostate Cancer.
- Asim M, Tarish F, Zecchini HI, et al. Synthetic lethality between androgen receptor signalling and the PARP pathway in prostate cancer. Nat Commun. 2017; 8:374.
- Schiewer MJ, Goodwin JF, Han S, et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov. 2012; 2:1134-1149.
- Li L, Karanika S, Yang G, et al. Androgen receptor inhibitor-induced “BRCAness” and PARP inhibition are synthetically lethal for castration-resistant prostate cancer. Sci Signal. 2017;10: eaam7479.
- Clarke NW, Armstrong AJ, Thiery-Vuillemin A, et al. Abiraterone and Olaparib for Metastatic Castration-Resistant Prostate Cancer. NEJM Evidence. 2022;1(9).
- Saad F, Clarke NW, Oya M, et al. Olaparib plus abiraterone versus placebo plus abiraterone in metastatic castration-resistant prostate cancer (PROpel): final prespecified overall survival results of a randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24(10):1094-1108.
- Chi KN, Rathkopf D, Smith MR, et al. Niraparib and Abiraterone Acetate for Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol. 2023;41(18):3339-3351.
- Chi KN, Sandhu S, Smith MR, et al. Niraparib plus abiraterone acetate with prednisone in patients with metastatic castration-resistant prostate cancer and homologous recombination repair gene alterations: second interim analysis of the randomized phase III MAGNITUDE trial. Ann Oncol. 2023;34(9):772-782.
- Agarwal N, Azad AA, Carles J, et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): a randomised, placebo-controlled, phase 3 trial. Lancet. 2023;402(10398):291-303.
- Fizazi K, Azad AA, Matsubara N, et al. First-line talazoparib with enzalutamide in HRR-deficient metastatic castration-resistant prostate cancer: the phase 3 TALAPRO-2 trial. Nat Med. 2024;30(1):257-264.
- Schaeffer EM, Srinivas S, Adra N, et al. Prostate Cancer, Version 4.2023, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw. 2023;21(10):1067-1096.
- Kurian AW, Abrahamse P, Furgal A, et al. Germline Genetic Testing After Cancer Diagnosis. JAMA. 2023;330(1):43-51.
- Zhen JT, Syed J, Nguyen KA, et al. Genetic testing for hereditary prostate cancer: Current status and limitations. Cancer. 2018;124(15):3105-3117.
The Current Landscape of Metastatic Castration-Resistant Prostate Cancer: Immunotherapy and Targeted Therapies
- Written by: Rashid Sayyid, MD, MSc, & Zachary Klaassen, MD, MSc
- References:
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411-422.
- FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-pembrolizumab-first-tissuesite-agnostic-indication. Accessed on Aug 6, 2022.
- Abida W, Cheng ML, Armenia J, et al. Analysis of the prevalence of microsatellite instability in prostate cancer and response to immune checkpoint blockade. JAMA Oncol. 2019;5(4):471-478.
- Pritchard CC, Morrissey C, Kumar A,et al. Complex MSH2and MSH6mutations in hypermutated microsatellite unstable advanced prostate cancer. Nat Commun. 2014;5:4988.
- National Comprehensive Cancer Network . Prostate Cancer (Version 4.2022). https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed Aug 6, 2022.
- Antonarakis ES, Piulats JM, Gross-Goupil M, et al. Pembrolizumab for Treatment-Refractory Metastatic Castration-Resistant Prostate Cancer: Multicohort, Open-Label Phase II KEYNOTE-199 Study. J Clin Oncol. 2020;38(5):395-405.
- Rubin MA, Maher CA, Chinnaiyan AM. Common gene rearrangements in prostate cancer. J Clin Oncol. 2011;29(27):3659-3668.
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N Engl J Med. 20116;375:443-453.
- Kunkel TA, Erie DA. DNA mismatch repair. Annu Rev Biochem. 2005;74:681-710.
- Abida W, Patnaik A, Campbell D, et al. Rucaparib in Men With Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J Clin Oncol. 2020;38(32):3763-3772.
- Abida W, Campbell D, Patnaik A, et al. Non-BRCA DNA Damage Repair Gene Alterations and Response to the PARP Inhibitor Rucaparib in Metastatic Castration-Resistant Prostate Cancer: Analysis From the Phase II TRITON2 Study. Clin Cancer Res. 2020;26(11):2487-96.
- Hussain M, Mateo J, Fizazi K, et al. Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020;383:2345-57.
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020;382:2091-2102.
- Thiery-Vuillemin A, de Bono J, Hussain M, et al. Pain and health-related quality of life with olaparib versus physician's choice of next-generation hormonal drug in patients with metastatic castration-resistant prostate cancer with homologous recombination repair gene alterations (PROfound): an open-label, randomised, phase 3 trial. Lancet Oncol. 2022;23(3):393-405.
- de Bono JS, Mehra N, Scagliotti GV, et al. Talazoparib monotherapy in metastatic castration-resistant prostate cancer with DNA repair alterations (TALAPRO-1): an open-label, phase 2 trial Lancet Oncol. 2021;22(9):1250-1264.
- Gasmi A, Roubaud G, Dariane C, et al. Overview of the Development and Use of Akt Inhibitors in Prostate Cancer. J Clin Med. 2022;11(1):160.
- De Bono JS, De Giorgi U, Rodrigues DN, et al. Randomized Phase II Study Evaluating Akt Blockade with Ipatasertib, in Combination with Abiraterone, in Patients with Metastatic Prostate Cancer with and without PTEN Loss. Clin Cancer Res. 2019;25:928–936.
- Sweeney C, Bracarda S Sternberg CN, et al. Ipatasertib plus abiraterone and prednisolone in metastatic castration-resistant prostate cancer (IPATential150): a multicentre, randomised, double-blind, phase 3 trial. Lancet. 2021;398(10295):131-142.
- Crabb SJ, Griffiths G, Marwood E, et al. Pan-AKT Inhibitor Capivasertib With Docetaxel and Prednisolone in Metastatic Castration-Resistant Prostate Cancer: A Randomized, Placebo-Controlled
PARP Inhibitor Therapy for Prostate Cancer Patients: Emerging Combinations
Introduction
Poly (adenosine diphosphate-ribose) polymerase (PARP) inhibitors are drugs that prevent the repair of DNA single-stranded breaks and promote their conversion to double-stranded breaks resulting in a synthetic lethality.1 These drugs have demonstrated promising results for the treatment of metastatic castrate-resistant prostate cancer (mCRPC) patients who experience disease progression following prior androgen receptor pathway inhibitor (ARPI) and/or taxane-based chemotherapy.There is growing interest in combining these agents with other classes of drugs that may have synergistic mechanisms of action. A prime example of this is the use of combination PARP inhibitors and APRIs, with ARPIs inhibiting the transcription of specific homologous recombination repair (HRR) genes, inducing an HRR deficiency-like state, which potentiates PARP inhibitor activity, and, conversely, PARP inhibitors upregulating androgen receptor signaling, enhancing ARPI activity.2-4 This has culminated in the approval of three PARP inhibitor/ARPI combinations by the US Food and Drug Administration (FDA) for the treatment of mCRPC patients in the first line setting:
- Olaparib plus abiraterone for BRCA1/2-mutated patients5
- Niraparib plus abiraterone for BRCA1/2-mutated patients6
- Talazoparib plus enzalutamide for HRR-mutated patients7
PARP Inhibitors + Radium-223
Olaparib + Radium-223For patients with bone metastases, it has been theorized that the combination of a PARP inhibitor and radium-223 may have synergistic mechanisms of action. PARP inhibitors have shown efficacy as radiosensitizing agents which may promote the efficacy of radium-223, an α-emitting radioisotope that induces DNA double-strand breaks leading to cell death. This formed the foundation for the COMRADE trial, an open-label, multi-center, phase 1/2 study trial to test the safety and efficacy of radium-223 and olaparib. This trial included men with mCRPC who had ≥2 bone metastases without evidence of concurrent visceral metastases or lymphadenopathy > 4 cm.
The phase 1 portion of the study employed a 3+3 dose escalation design with fixed-dose radium-223 (55 kBq/kg IV every 4 weeks x 6) and escalating doses of olaparib. The dose level 1 (DL1) for was olaparib 200 mg PO BID while DL2 was 300 mg PO BID. In phase 1, the primary objective was to determine the recommended phase 2 dose (RP2D) for the randomized portion of the study, which was found to be 200 mg BID for olaparib. No dose limiting toxicities were observed at either DL1 or DL2. However, 5 of 6 patients enrolled at DL2 required dose reduction. Assessing secondary objectives, the authors found that the PSA response and alkaline phosphatase response rates were 16.7% (n=2) and 67% (n=8), respectively. At a median follow-up of 6.5 months, the 6 months rPFS was 58%, and the 12 months OS was 56%. Based on these results, the investigators concluded that olaparib can be safely combined with radium-223 at the RP2D of 200 mg orally twice daily with fixed dose radium-223.8
Niraparib + Radium-223
Utilizing a similar treatment strategy to that seen in the COMRADE trial, the combination of niraparib and radium-223 was evaluated in the phase 1b trial, NiraRad. This trial included 30 men with progressive mCRPC following ≥1 line of an ARPI and had evidence of bone metastases without bulky visceral disease and no documented BRCA1/2 alterations. The niraparib dose was escalated in combination with standard dosing of Radium-223 using a time-to-event continual reassessment method. The investigators determined that for patients with prior chemotherapy exposure, the maximum tolerated dose (MTD) for niraparib was 100 mg, whereas the MTD for chemotherapy-naïve patients was 200 mg. The median rPFS for all patients included in analysis was 7.1 months with an estimated 6-month rPFS of 51%.9
PARP Inhibitors + 177Lu-PSMA-617
177Lu-PSMA-617 delivers significant beta radiation to PSMA-expressing tumors causing single strand DNA breaks, which are typically repaired by PARP-dependent pathways. Blocking the PARP enzyme could have a synergistic mechanism of action by converting DNA single strand breaks to lethal double strand breaks via replication fork collapse. In the LuPARP trial presented at ASCO 2023, the investigators hypothesized that olaparib would promote the radiosensitization of 177Lu-PSMA-617, resulting in intensification of DNA damage and, thus, improved efficacy.
The LuPARP phase 1 trial schema was as follows:

This trial included 48 patients with mCRPC, and all eligible patients had received a prior ARPI and docetaxel. All patients underwent a 68Ga-PSMA-11 plus an FDG-PET/CT with the following inclusion criteria:
- PSMA SUVmax >15 at any site
- SUVmax >10 at other sites
- No FDG discordance
From an efficacy standpoint, 177Lu-PSMA-617 in combination with olaparib demonstrated promising activity: in the overall cohort (i.e., Cohorts 1 to 9), the PSA50 and PSA90 response rates were 66% and 44%, respectively. The objective response rate (ORR) by RECIST v1.1 criteria was 78%.10 Compared to the results of the TheraP and VISION trials, the PSA50 responses were identical to those from TheraP (66%) and higher than those in VISION (46%).11,12 The PSA90 response of 44% in LuPARP was slightly higher than that in TheraP (38%).
Moreover, early results from Cohorts 7-9 were promising with PSA50 and PSA90 responses of 75% and 58%, respectively. However, results from this Phase 1 trial are not designed, nor powered, to assess efficacy outcomes.

PARP Inhibitors + Immune Checkpoint Inhibitors
While immunotherapy has shown limited success in the mCRPC disease space, it is hypothesized that the increased cellular DNA damage induced by PARP inhibitors may lead to increased immune priming and subsequently promote immune cell infiltration. This has served as the rationale for emerging trials of combination PARP inhibitors and immune checkpoint inhibitors.Rucaparib + Nivolumab
The CheckMate 9KD trial has evaluated the combination of rucaparib and nivolumab in two cohorts:
- Cohort A1: Post-chemotherapy mCRPC (1–2 taxanes and ≤2 ARPIs)
- Cohort A2: Chemotherapy-naïve mCRPC (Received prior ARPI)
Among patients in Cohort A1 (n=58), the ORR was 10.3% in the overall cohort. Superior ORRs were observed in the HRD-positive (17.2%) and BRCA1/2-positive tumors (33.3%). PSA50 responses were observed in 12% of patients in the overall cohort, compared to 18% and 42% of HRD-positive and BRCA1/2-positive tumors, respectively. Median rPFS ranged between 4.9 and 5.8 months, whereas OS ranged between 13.9 and 15.4 months.

As expected, response rates and survival outcomes were superior in the less heavily pre-treated Cohort A2 (n=39). The ORR was 15.4% in the overall cohort, with ORRs of 25% and 33.3% in the HRD-positive and BRCA1/2-positive tumors, respectively. PSA50 responses were observed in 27.3% of patients in the overall cohort, compared to 42% and 85% of HRD-positive and BRCA1/2-positive tumors, respectively. Median rPFS ranged between 8.1 and 10.9 months, whereas OS ranged between 20.2 and 22.7 months.

In cohorts A1 and A2, respectively, the most common any-grade and grade 3–4 treatment-related adverse events were nausea (41%) and anemia (14–21%). Approximately 25% of patients discontinued treatment secondary to adverse events.13
Olaparib + Pembrolizumab
Cohort A of the phase 1b/2 KEYNOTE-365 study enrolled patients with molecularly unselected, docetaxel-pretreated mCRPC whose disease progressed within 6 months of screening. In this trial, 102 patients received pembrolizumab 200 mg IV every 3 weeks + olaparib 400 mg capsule or 300 mg tablet orally twice daily. Patients could have received one chemotherapy agent other than docetaxel for mCRPC and ≤2 ARPIs. The primary endpoints were PSA50 response rates, ORR, and safety.
A PSA50 response was observed in 15% of patients. The confirmed ORR was 8.5% (5 partial responses) among patients with measurable disease.

The median rPFS was 4.5 months, and the median OS was 14 months. Treatment-related adverse events were observed in 91% of patients. Grade 3–5 events occurred in 48% of patients (6% deaths), most commonly anemia (27%), fatigue (6%), and neutropenia (5%).14
This combination of olaparib + pembrolizumab was next assessed in the open-label, phase III KEYLYNK-010 trial that randomized mCRPC patients that had progressed on one prior ARPI and docetaxel in a 2:1 fashion to olaparib + pembrolizumab versus the alternate ARPI (i.e., if had received abiraterone, given enzalutamide and vice versa). The dual primary endpoints were rPFS and OS. This trial included 793 patients of whom 529 and 264 were randomized to olaparib + pembrolizumab and an alternate ARPI, respectively. There was no significant difference in rPFS (median: 4.4 versus 4.2 months; HR: 1.02, 95% CI: 0.82 – 1.25, p=0.55) or OS between the two treatment arms (median 15.8 versus 14.6 months; HR: 0.94, 95% CI: 0.77 – 1.14, p=0.26).

Grade 3 treatment-related adverse events were more common with olaparib + pembrolizumab (35% versus 9%), with events leading to treatment discontinuation occurring in 11% and 1.6% of patients in the intervention and control arms, respectively. The most common grade ≥3 adverse events with olaparib + pembrolizumab were anemia (20%), fatigue (3%), and asthenia (2.3%).15
Olaparib + Durvalumab
In a single arm phase II trial, the combination of durvalumab 1,500 mg IV every 4 weeks and olaparib 300 mg twice daily was evaluated in 17 mCRPC patients with disease progression following prior ARPI. Overall, 9/17 (53%) patients had a PSA50 response, with 4 of these 9 patients having a radiographic response. The median rPFS of patients with DDR gene alteration was 16.1 months, with a 12-months PFS probability of 83.3%, compared to 36.4% in those without mutations (p=0.031). The most common treatment-related grade 3 or 4 adverse events were anemia (24%), lymphopenia (12%), infection (12%), and nausea (12%).16

Talazoparib + Avelumab
The JAVELIN PARP Medley trial is a phase 1b/2 basket trial evaluating the combination of talazoparib and avelumab in patients with advanced solid tumors, including mCRPC patients with and without HHR alterations (n=21). Patients received avelumab 800 mg every 2 weeks plus talazoparib 1mg once daily. In the overall cohort, PSA responses were observed in 2/21 patients, and in the HRR positive mCRPC cohort, the ORR was 11.1%.17
PARP Inhibitors + Bipolar Androgen Therapy
Prostate cancer cells can develop resistance to androgen ablation through an adaptive marked upregulation of androgen receptors over time in response to a low-androgen milieu. This upregulation can make these cells vulnerable to supraphysiologic testosterone exposure. Bipolar Androgen Therapy (BAT) has been proposed as a technique to overcome AR therapeutic resistance. Rapid cycling between polar extremes of supraphysiologic and near-castrate serum testosterone in asymptomatic men with mCRPC has proven to be safe and effective.18Supraphysiologic androgen levels have been shown to induce double-strand DNA breaks and suppress the expression of genes involved in the DNA repair process.19,20 This has served as the rationale for evaluating the combination of olaparib and BAT in a single arm phase II trial. Thirty-six patients with mCRPC and disease progression following abiraterone and/or enzalutamide received olaparib 300 mg twice daily plus BAT (testosterone cypionate/enanthate 400 mg every 28 days with ongoing androgen deprivation). A PSA50 response was observed in 11/36 patients (31%) at 12 weeks, and the median rPFS in the intent-to-treat cohort was 13 months. The most frequently observed treatment-related adverse events were gastrointestinal related and fatigue. Five patients had grade ≥3 treatment-related adverse events, including one stroke (Grade 4) and one myocardial infarction (Grade 5).21

PARP Inhibitors + Chemotherapy
The combination of the low dose oral PARP inhibitor veliparib (ABT-888) and temozolomide for docetaxel pre-treated mCRPC patients was evaluated in a single-arm, open-label, pilot study published by Hussain et al. in 2014. This trial included 26 patients with a median baseline PSA of 170 ng/ml. A PSA response was observed in 2 patients (8%), with a further 13 having stable PSA levels. The median PFS was 9 weeks, and the median OS was 40 weeks. Grade 3/4 adverse events occurred in >10 % of patients include thrombocytopenia (23 %) and anemia (15 %).22PARP Inhibitors + Targeted Therapies
Olaparib + CediranibCediranib is a pan-vascular endothelial growth factor receptor inhibitor that suppresses the expression of HRR genes and increases sensitivity to PARP inhibition in preclinical models.23 In an open-label phase II trial, patients with progressive mCRPC were randomly assigned to receive either cediranib 30 mg once daily plus olaparib 200 mg twice daily versus olaparib 300 mg twice daily alone. In the intention-to-treat cohort of 90 patients, the median rPFS was 8.5 months in the combination arm versus 4 months in the PARP inhibitor monotherapy arm (HR 0.62; 95% CI: 0.39–0.97, p=0.036). Among patients with HRR-deficient mCRPC, the median rPFS was 10.6 months with combination treatment versus 3.8 months with olaparib monotherapy. In the subset of patients with BRCA2-mutated mCRPC, median rPFS was 13.8 months in the combination arm versus 11.3 months in the olaparib only arm. Grade 3–4 adverse events occurred in 61% of patients in the combination arm, compared to 18% of patients in the monotherapy arm.24

Olaparib + Ceralasterib
In an in vitro study, the combination of olaparib and the ataxia telangiectasia and Rad3-related protein (ATR) inhibitor, ceralasterib, was shown to selectively cause cell death in ATM-deficient cells.25 This served as the basis for the TRAP trial, a two-cohort study of mCRPC patients with HRR mutations (BRCA1/2 or ATM; n=35) and another without HRR mutations (n=12). All patients had progressed on ≥1 prior mCRPC therapy with no prior PARP inhibitors or platinum chemotherapy. In this study, olaparib was administered twice daily at a standard dose, and ceralasterib was administered daily on days 1¬–7 of a 28-day cycle. The primary endpoint was disease response (confirmed PSA50 or RECIST response). The response rate in the HRR cohort was 33%, compared to 11% in the HRR negative cohort, including 21% of patients experiencing a grade 3 treatment-related adverse event (no grade 4–5 events).26

Conclusions and Future Trials
PARP inhibitors are an exciting class of drugs with a unique mechanism of action that lends itself to potential synergistic combinations with other classes of drugs. To date, the only combination to receive regulatory approval is that of PARP inhibitors + ARPIs; however, numerous exciting combinations continue to emerge. Additionally, given their success in the mCRPC disease space, there is increased interest in evaluating such combinations in earlier disease stages, including the high-risk localized and the metastatic hormone-sensitive settings. Summarized in the table below are select trials of PARP inhibitor combination therapy across the prostate cancer spectrum.

Published March 2024
- Written by: Zachary Klaassen, MD, MSc Associate Professor of Urology Urologic Oncologist Medical College of Georgia, Georgia Cancer Center Augusta, GA and Rashid Sayyid, MD, MSc Urologic Oncology Fellow University of Toronto Toronto, Ontario, Canada
- References:
- Xie T, Dickson K, Yee C, et al. Targeting Homologous Recombination Deficiency in Ovarian Cancer with PARP Inhibitors: Synthetic Lethal Strategies That Impact Overall Survival. Cancers (Basel). 2022;14(19):4621.
- Asim M, Tarish F, Zecchini HI, et al. Synthetic lethality between androgen receptor signalling and the PARP pathway in prostate cancer. Nat Commun. 2017; 8:374.
- Schiewer MJ, Goodwin JF, Han S, et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov. 2012; 2:1134-49.
- Li L, Karanika S, Yang G, et al. Androgen receptor inhibitor-induced “BRCAness” and PARP inhibition are synthetically lethal for castration-resistant prostate cancer. Sci Signal. 2017;10: eaam7479.
- FDA approves olaparib with abiraterone and prednisone (or prednisolone) for BRCA-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-olaparib-abiraterone-and-prednisone-or-prednisolone-brca-mutated-metastatic-castration. Accessed on March 10, 2024.
- FDA approves niraparib and abiraterone acetate plus prednisone for BRCA-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-niraparib-and-abiraterone-acetate-plus-prednisone-brca-mutated-metastatic-castration. Accessed on March 10, 2024.FDA approves niraparib and abiraterone acetate plus prednisone for BRCA-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-niraparib-and-abiraterone-acetate-plus-prednisone-brca-mutated-metastatic-castration. Accessed on March 10, 2024.FDA approves niraparib and abiraterone acetate plus prednisone for BRCA-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-niraparib-and-abiraterone-acetate-plus-prednisone-brca-mutated-metastatic-castration. Accessed on March 10, 2024.
- FDA approves talazoparib with enzalutamide for HRR gene-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-talazoparib-enzalutamide-hrr-gene-mutated-metastatic-castration-resistant-prostate. Accessed on March 10, 2024.FDA approves talazoparib with enzalutamide for HRR gene-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-talazoparib-enzalutamide-hrr-gene-mutated-metastatic-castration-resistant-prostate. Accessed on March 10, 2024.FDA approves talazoparib with enzalutamide for HRR gene-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-talazoparib-enzalutamide-hrr-gene-mutated-metastatic-castration-resistant-prostate. Accessed on March 10, 2024.
- Pan E, Xie W, Ajmera A, et al. A Phase I Study of Combination Olaparib and Radium-223 in Men with Metastatic Castration-Resistant Prostate Cancer (mCRPC) with Bone Metastases (COMRADE). Mol Cancer Ther. 2023;22(4):511-518.
- Quinn Z, Leiby B, Sopavde G, et al. Phase I Study of Niraparib in Combination with Radium-223 for the Treatment of Metastatic Castrate-Resistant Prostate Cancer. Clin Cancer Res. 2023;29(1):50-59.
- Sandhu S, Joshua AM, Emmett L, et al . LuPARP: Phase 1 trial of 177Lu-PSMA-617 and olaparib in patients with metastatic castration resistant prostate cancer (mCRPC). J Clin Oncol. 2023;41(16):Suppl 5005.
- Sartor O, de Bono J, Chi KN, et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021;385:1901-1103
- Hofman MS, Emmett L, Sandhu S, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397(10276):797-804.
- Fizazi K, Retz M, Petrylak DP, et al. Nivolumab plus rucaparib for metastatic castration-resistant prostate cancer: results from the phase 2 CheckMate 9KD trial. J Immunother Cancer. 2022;10(8):e004761.
- Yu EY, Piulats JM, Gravis G, et al. Pembrolizumab plus Olaparib in Patients with Metastatic Castration-resistant Prostate Cancer: Long-term Results from the Phase 1b/2 KEYNOTE-365 Cohort A Study. Eur Urol. 2023;83(1):15-26.
- Antonarakis ES, Park SH, Goh JC, et al. Pembrolizumab Plus Olaparib for Patients With Previously Treated and Biomarker-Unselected Metastatic Castration-Resistant Prostate Cancer: The Randomized, Open-Label, Phase III KEYLYNK-010 Trial. J Clin Oncol. 2023;41(22):3839-3850.
- Karzai F, VanderWeele D, Madan RA, et al. Activity of durvalumab plus olaparib in metastatic castration-resistant prostate cancer in men with and without DNA damage repair mutations. J Immunother Cancer. 2018;5(1):141.
- Yap TA, Bardia A, Dvorkin M, et al. Avelumab Plus Talazoparib in Patients With Advanced Solid Tumors: The JAVELIN PARP Medley Nonrandomized Controlled Trial. JAMA Oncol. 2023;9(1):40-50.
- Denmeade SR, Wang H, Agarwal N, et al. TRANSFORMER: A Randomized Phase II Study Comparing Bipolar Androgen Therapy Versus Enzalutamide in Asymptomatic Men With Castration-Resistant Metastatic Prostate Cancer. J Clin Oncol. 2021;39(12):1371-1382.
- Haffner MC, Aryee MJ, Toubaji A, et al. Androgen-induced TOP2B-mediated double-strand breaks and prostate cancer gene rearrangements. Nat Genet. 2010;42(8):668-675.
- Chatterjee P, Schweizer MT, Lucas JM, et al. Supraphysiological androgens suppress prostate cancer growth through androgen receptor-mediated DNA damage. J Clin Invest. 2019;129(1):4245-4260.
- Schweizer MT, Gulati R, Yezefski T, et al. Bipolar androgen therapy plus olaparib in men with metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 2023;26(1):194-200.
- Hussain M, Carducci MA, Slovin S, et al. Targeting DNA repair with combination veliparib (ABT-888) and temozolomide in patients with metastatic castration-resistant prostate cancer. Invest New Drugs. 2014;32(5):904-912.
- Kaplan AR, Gueble SE, Liu Y, et al. Cediranib suppresses homology-directed DNA repair through down-regulation of BRCA1/2 and RAD51. Sci Transl Med. 2019;11:eaav4508.
- Kim JW, McKay RR, Radke MR, et al. Randomized Trial of Olaparib With or Without Cediranib for Metastatic Castration-Resistant Prostate Cancer: The Results From National Cancer Institute 9984. J Clin Oncol. 2023;41(4):871-880.
- Lloyd RL, Wijnhoven PWG, Ramos-Montoya A, et al. Combined PARP and ATR inhibition potentiates genome instability and cell death in ATM-deficient cancer cells. Oncogene. 2020;39(25):4869-4883.
- Reichert ZR, Devitt ME, Alumkal JJ, et al. Targeting resistant prostate cancer, with or without DNA repair defects, using the combination of ceralasertib (ATR inhibitor) and olaparib (the TRAP trial). J Clin Oncol. 2022;40(6):Supplement.
The Current Landscape of Metastatic Castration-Resistant Prostate Cancer: Novel Hormonal Therapies
While the emergence of castration resistant disease comes as a result of the disease progressing in spite of castrate levels of testosterone (at times called hormone refractory disease), prostate cancer (even in the castration resistance prostate cancer (CRPC) setting) remains heavily dependent on the androgen axis.
- Written by: Rashid Sayyid, MD MSc, & Zachary Klaassen, MD MSc
- References:
- de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995-2005
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138-148.
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. New Engl J Med. 2012;367(13):1187-1197.
- Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424-433.
- Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in Men with Chemotherapy-naive Metastatic Castration-resistant Prostate Cancer: Extended Analysis of the Phase 3 PREVAIL Study. Eur Urol. 2016.
- Denmeade SR, Wang H, Agarwal N, et al. TRANSFORMER: A Randomized Phase II Study Comparing Bipolar Androgen Therapy Versus Enzalutamide in Asymptomatic Men With Castration-Resistant Metastatic Prostate Cancer. J Clin Oncol. 2021;39(12):1371-1382.
- Bipolar Androgen Therapy in Men with Metastatic Castration-resistant Prostate Cancer (RESTORE): A Comparison of Post-abiraterone Versus Post-enzalutamide Cohorts. Eur Urol. 2021;692-699.
- Khalaf DJ, Annala M, Taavitsainen S, et al. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label, phase 2, crossover trial. Lancet Oncol. 2019l20(12):1730-1739.
- Saad F, Efstathiou E, Attard G, et al. Apalutamide plus abiraterone acetate and prednisone versus placebo plus abiraterone and prednisone in metastatic, castration-resistant prostate cancer (ACIS): a randomised, placebo-controlled, double-blind, multinational, phase 3 study. Lancet Oncol. 2021;22(11):1541-1559.
- Colomba E, Jonas SF, Eymard J-C, et al. Objective computerized cognitive assessment in men with metastatic castrate-resistant prostate cancer (mCRPC) randomly receiving darolutamide or enzalutamide in the ODENZA trial. Ann Oncol. 2021;32(5):S66-647.
- Cathomas R, Procopio G, Hayoz S, et al. Darolutamide maintenance in metastatic castration resistant prostate cancer (mCRPC) previously treated with novel hormonal agents (NHA) and non-progressive disease after subsequent treatment with a taxane: A randomized double-blind placebo-controlled phase II trial (SAKK 08/16). Ann Oncol. 2021;32(5):S1301-1302.
PARP Inhibitor Monotherapy for Prostate Cancer Patients
Introduction
Over the past decade, there have been significant advances in defining the genomic landscape of prostate cancer. The landmark study by Pritchard et al. published in The New England Journal of Medicine in 2016 demonstrated that germline DNA-repair gene mutations were present in approximately 12% of metastatic prostate cancer patients, most commonly BRCA2 (5.3%), CHEK2 (1.9%), and ATM (1.6%). Significantly, the frequency of such mutations increases across the prostate cancer spectrum – 2% in patients with NCCN localized low-to-intermediate risk tumors, 6% in those with localized high-risk tumors, and as high as 24% in patients with metastatic castrate-resistant prostate cancer (mCRPC).1 This is of utmost clinical importance as such mutations, both inherited and acquired (i.e., somatic), represent actionable clinical targets for drug therapy.
Poly (adenosine diphosphate-ribose) polymerase (PARP) inhibitors are drugs that prevent the repair of DNA single-stranded breaks and promote their conversion to double-stranded breaks leading to a synthetic lethality. These agents are most effective in homologous recombination repair (HRR)-deficient tumors (e.g., BRCA1/2), due to their compromised ability to repair DNA double strand breaks.2 In addition to breast and ovarian malignancies, PARP inhibitors have gained regulatory approval for the treatment of mCRPC patients:
- Rucaparib for BRCA1/2-mutated patients (FDA approved in 2020)3
- Olaparib for HRR-mutated patients (FDA approved in 2020)4
- Olaparib plus abiraterone for BRCA1/2-mutated patients (FDA approved in 2023)5
- Niraparib plus abiraterone for BRCA1/2-mutated patients (FDA approved in 2023)6
- Talazoparib plus enzalutamide for HRR-mutated patients (FDA approved in 2023)7
In this Center of Excellence article, we will provide an in-depth overview of the current evidence for PARP inhibitor monotherapy in prostate cancer, summarizing efficacy results from major trials and discussing the adverse event profile of these agents.
Current Evidence for PARP Inhibitor Monotherapy
Olaparib
TOPARP-A was a pivotal phase II trial of olaparib in mCRPC in which 50 patients were treated with olaparib 400 mg twice daily until disease progression.8 The primary endpoint was the composite response rate defined either as an objective response according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria, or a ≥ 50% reduction in prostate-specific antigen (PSA50), or a reduction in the circulating tumor-cell count from ≥ 5 per 7.5 ml of blood to < 5 per 7.5 ml. All patients had prior treatment with docetaxel and 49 (98%) with abiraterone or enzalutamide. Sixteen of 49 (33%) evaluable patients had a response. Overall, 14 of the 16 responders had homozygous deletions, deleterious mutations, or both in DNA-repair genes — including BRCA1/2, ATM, Fanconi’s anemia genes, and CHEK2.
This was followed by TOPARP-B, an open-label, phase II trial in which men with HRR-mutated mCRPC that had progressed on ≥1 taxane therapy were treated with olaparib 400 mg or 300 mg twice daily in a randomized fashion.9 The primary endpoint was identical to the TOPARP-A trial. A targetable HRR gene aberration was found in 161 of 592 (27.2%) patients who underwent a targeted next-generation tumor sequencing. However, sequencing could not be performed on 119 (17%) of consented patients because of insufficient or poor-quality tissue. The confirmed composite response rate was 54.3% in the 400 mg cohort and 39.1% in the 300 mg cohort (p=0.14). Median radiographic progression-free survival (rPFS) was 5.5 months (95% CI: 4.4 – 8.3) in the 400 mg cohort and 5.6 months (3.7 – 7.7) in the 300 mg cohort. The predefined criteria for success were met for the 400 mg regimen but not for the 300 mg regimen.
These promising results served as the ‘precursor’ for PROfound, a randomized, open-label, phase III trial of olaparib 300 mg twice daily versus physician’s choice of standard of care therapy in men with HRR-mutated mCRPC who had disease progression while receiving a novel hormonal agent (e.g., enzalutamide or abiraterone). Patients were assigned to one of two cohorts based on their HRR gene alteration. Cohort A included patients with BRCA1, BRCA2, or ATM alterations, irrespective of co-occurring alterations in any other HRR genes. Cohort B had patients with alterations in any of the other 12 HRR genes (BRIP1, BARD1, CDK12, CHEK 1/2, FANCL, PALB2, PPP2R2A, RAD51B, RAD51C, RAD51D, RAD54L). Patients within each cohort were randomized in 2:1 fashion to olaparib versus standard of care . The primary endpoint was the rPFS in cohort A.
Of the 4,425 enrolled patients, 4,047 had tumor tissue available for testing and only 2,792 (69%) were successfully sequenced. A qualifying alteration in one or more of the 15 HRR genes was detected in 778 of 2,792 patients (28%). Median rPFS was significantly longer in the olaparib group than in the standard of care group (7.4 months versus 3.6 months; HR: 0.34; 95% CI: 0.25 – 0.47; p<0.001).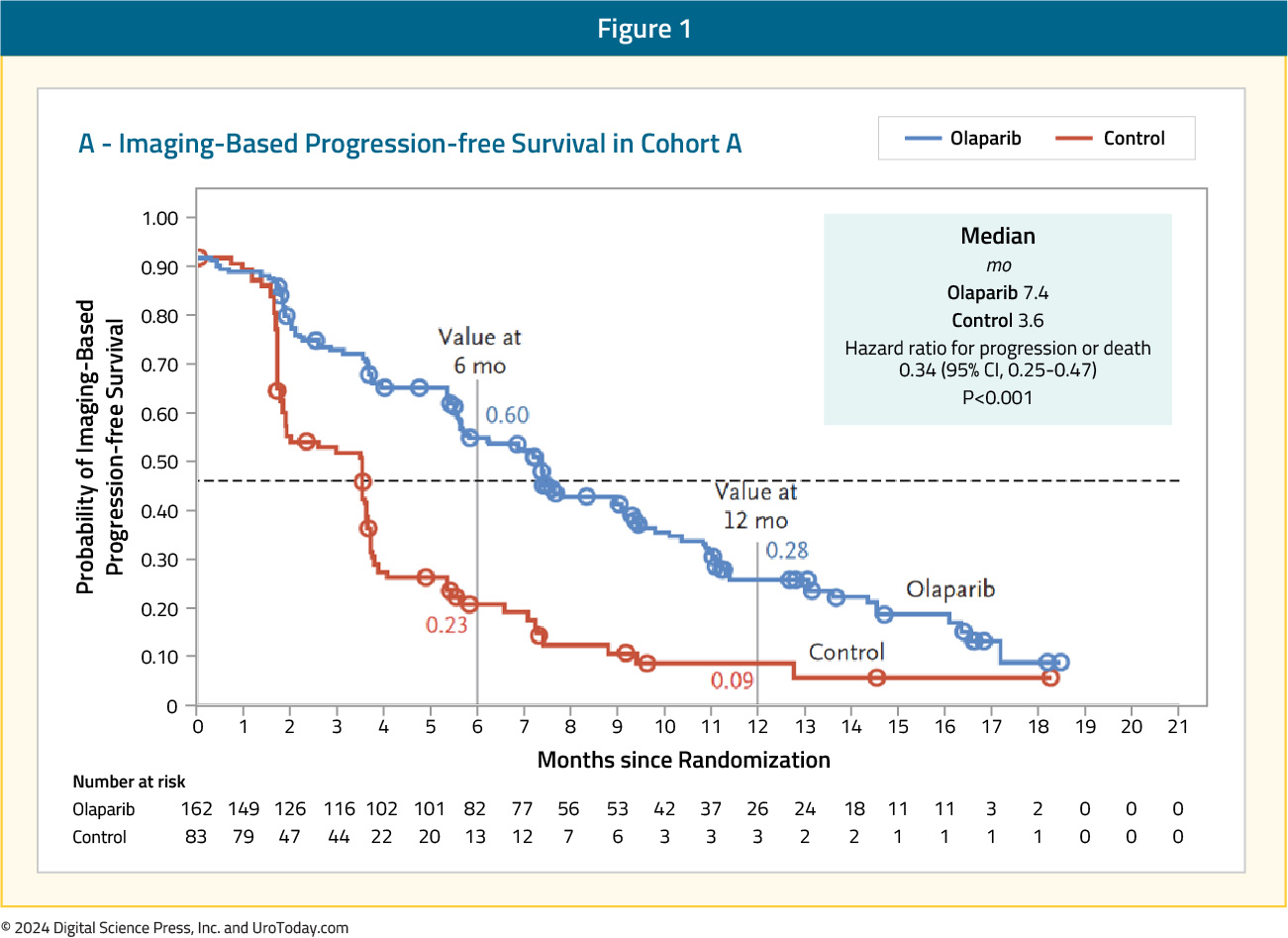
The confirmed objective response rate (ORR) was 33% in the olaparib group and 2% in the standard of care group (odds ratio 20.9; 95% CI: 4.2 – 379.2; p<0.001). The median time to pain progression was also significantly longer in the olaparib group (HR: 0.44; 95% CI: 0.22 – 0.91; p=0.02). The final overall survival (OS) analysis demonstrated that olaparib improved OS in cohort A from a median of 14.7 to 19.1 months (HR: 0.69, 95% CI: 0.50 – 0.97). Notably, 84% of patients with imaging-based disease progression had crossed over from the standard of care arm to olaparib at the time of analysis, which highlights the efficacy of earlier use of olaparib in this setting.10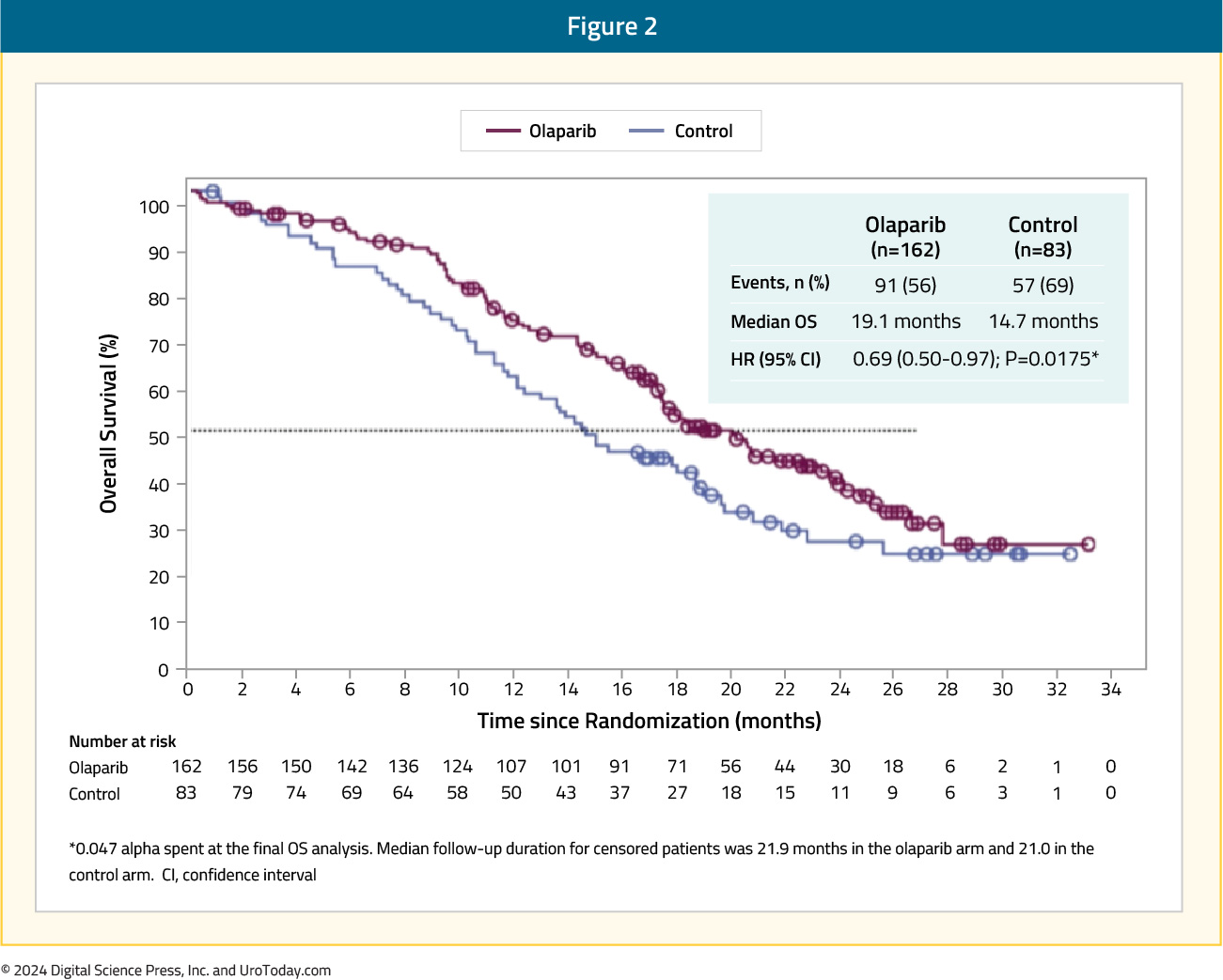
The data from PROfound formed the basis for the FDA-approval of olaparib 300 mg PO twice daily in men with HRR-mutated mCRPC after progression on enzalutamide or abiraterone.4
Rucaparib
The first PARP inhibitor to be approved by the FDA for the treatment of prostate cancer patients was rucaparib. On May 15, 2020, rucaparib was granted accelerated approval for patients with mCRPC and BRCA mutations (germline or somatic) who had progressed following treatment with androgen receptor-directed therapy and a taxane-based chemotherapy.3 This approval was based on the results of TRITON2, which was initially published in 202011 and most recently updated in 2023.12 TRITON2 is an international, open-label, phase II trial that evaluated the safety and efficacy of rucaparib 600 mg twice daily in mCRPC patients with DNA damage response (DDR) gene alterations who had progressed after 1–2 lines of an androgen receptor pathway inhibitor and one taxane-based chemotherapy. The efficacy cohort included 277 patients, of whom 172 (62.1%) had a deleterious germline or somatic BRCA alteration with 21.3%, 5.4%, 3.1%, 4%, and 4.7% having ATM, CDK12, CHEK2, PALB2, and other DDR gene mutations, respectively. A confirmed objective response was observed in 46% of BRCA patients with measurable disease (10% complete response). A superior response was observed among BRCA2 patients (48% versus 30% for BRCA1), which is potentially secondary to an increased frequency of biallelic mutations among BRCA2 patients and a greater coexistence of TP53 mutations among BRCA1-mutated men.13 The objective response was consistent irrespective of whether the BRCA mutation was somatic or germline and whether other DDR mutations were present or absent. All four patients with PALB2 mutations and measurable disease had an objective partial response, with none of the ATM-, CDK12-, CHEK2-mutated patients experiencing an objective response. A confirmed PSA50 response was observed in 53% and 55% of BRCA and PALB2-mutated patients, compared to 3.4–14% among patients with other DDR gene mutations. The median overall survival was 17.2 months for BRCA patients, compared to 11.1–14.6 months among ATM, CDK12, and CHEK2-mutated patients.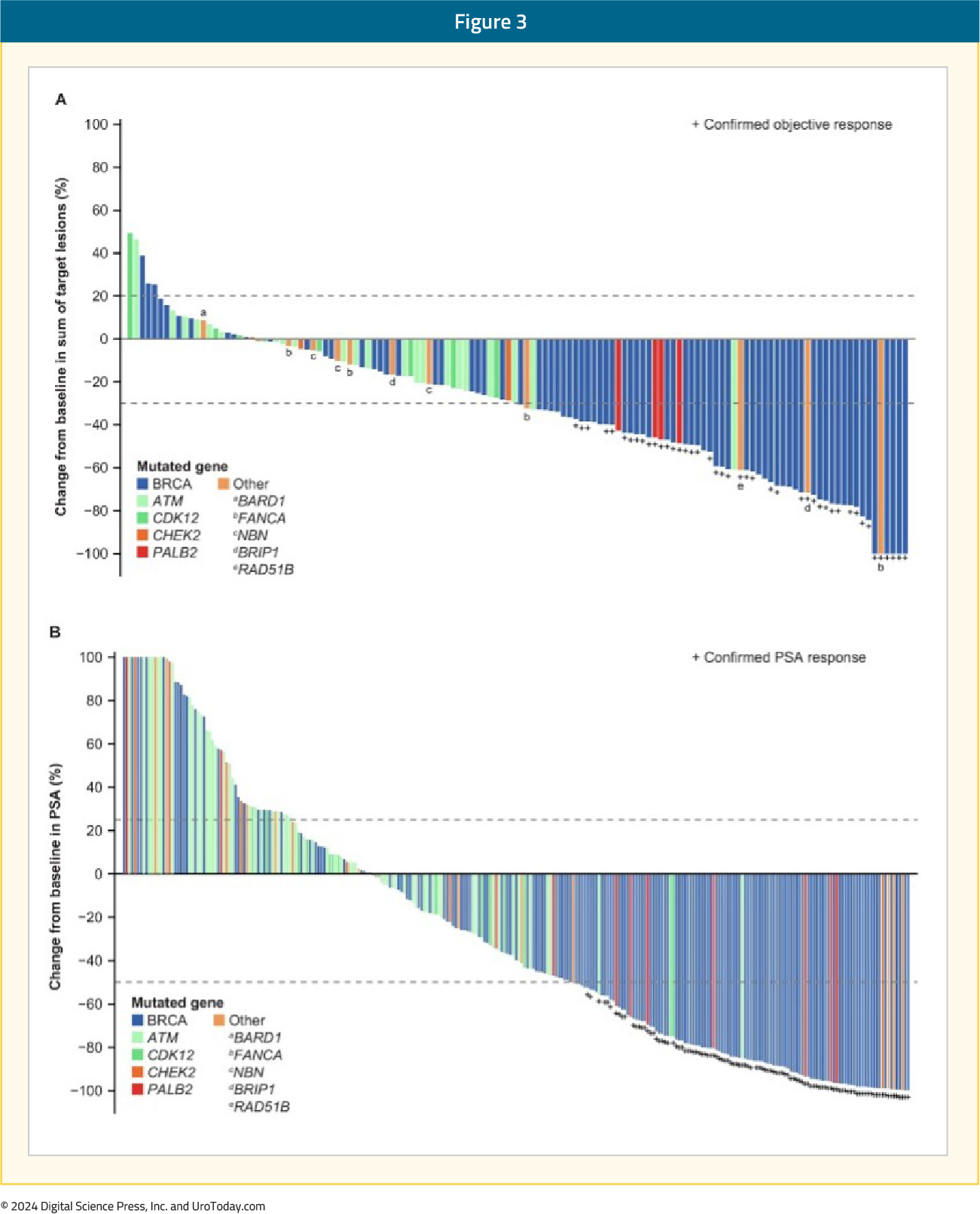
Following the promising results of TRITON2, the phase 3 TRITON3 trial was published in 2023. This was a randomized phase 3 trial of mCRPC patients with a BRCA1, BRCA2, or ATM alterations who experienced disease progression following treatment with a second-generation androgen receptor pathway inhibitor. Patients underwent 2:1 randomization to receive oral rucaparib (600 mg twice daily) or a physician’s choice control (docetaxel or a second-generation ARPI [abiraterone acetate or enzalutamide]). The primary outcome was the median PFS according to independent review. There were 405 patients randomized to receive rucaparib (n=270) or the control group (n=135). At 62 months follow-up, imaging-based PFS was significantly prolonged in the rucaparib group compared to the control group, both in the BRCA subgroup (11.2 and 6.4 months, respectively; HR: 0.50; 95% CI: 0.36 – 0.69) and in the intention-to-treat population (10.2 and 6.4 months, respectively; HR: 0.61; 95% CI: 0.47 – 0.80; p<0.001 for both comparisons). No significant PFS benefit was observed in the ATM subgroup.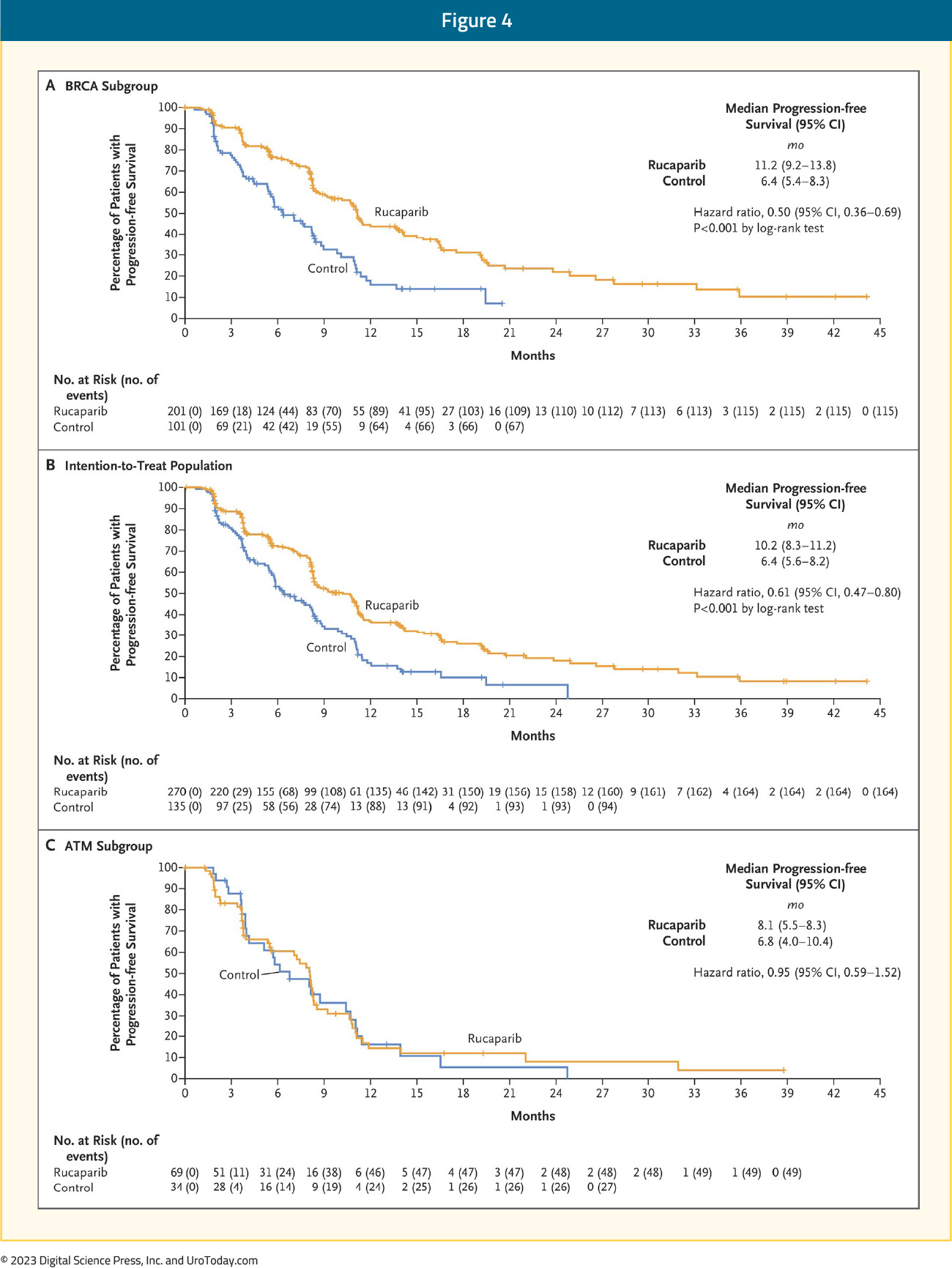
In the BRCA subgroup, the median OS was 24.4 versus 20.8 months in favor of rucaparib (HR: 0.81, 95% CI: 0.58 – 1.12, p=0.21).14
Talazoparib
TALAPRO-1 was an open-label, phase II trial that evaluated talazoparib 1 mg/day in patients with evidence of progressive mCRPC who had measurable soft-tissue disease and evidence of one of 11 DDR mutations who had progressed following taxane-based chemotherapy (48% both docetaxel and cabazitaxel) and abiraterone and/or enzalutamide (98% of population). The primary endpoint was confirmed ORR. There were 128 patients enrolled, of whom 127 received at least one dose of talazoparib (safety population) and 104 had measurable soft-tissue disease (antitumor activity population). After a median follow-up of 16.4 months, the ORR was 30% (95% CI: 21.2 – 39.6%).15
Niraparib
GALAHAD was a multicenter, open-label, single arm phase II trial of 289 mCRPC patients with DNA repair gene defects and disease progression following a prior next-generation androgen signaling inhibitor and a taxane, who received niraparib 300 mg orally once daily. The primary endpoint was ORR in patients with BRCA alterations and measurable disease. At a median follow-up of 10 months, the ORR in the measurable BRCA cohort was 34.2%. The median duration of objective response was 5.6 months. Conversely, the ORR in the measurable non-BRCA cohort was 10.6%. Median rPFS (8.1 versus 3.7 months) and OS (13.0 and 9.6 months) were both longer in the BRCA cohort, compared to the non-BRCA cohort.16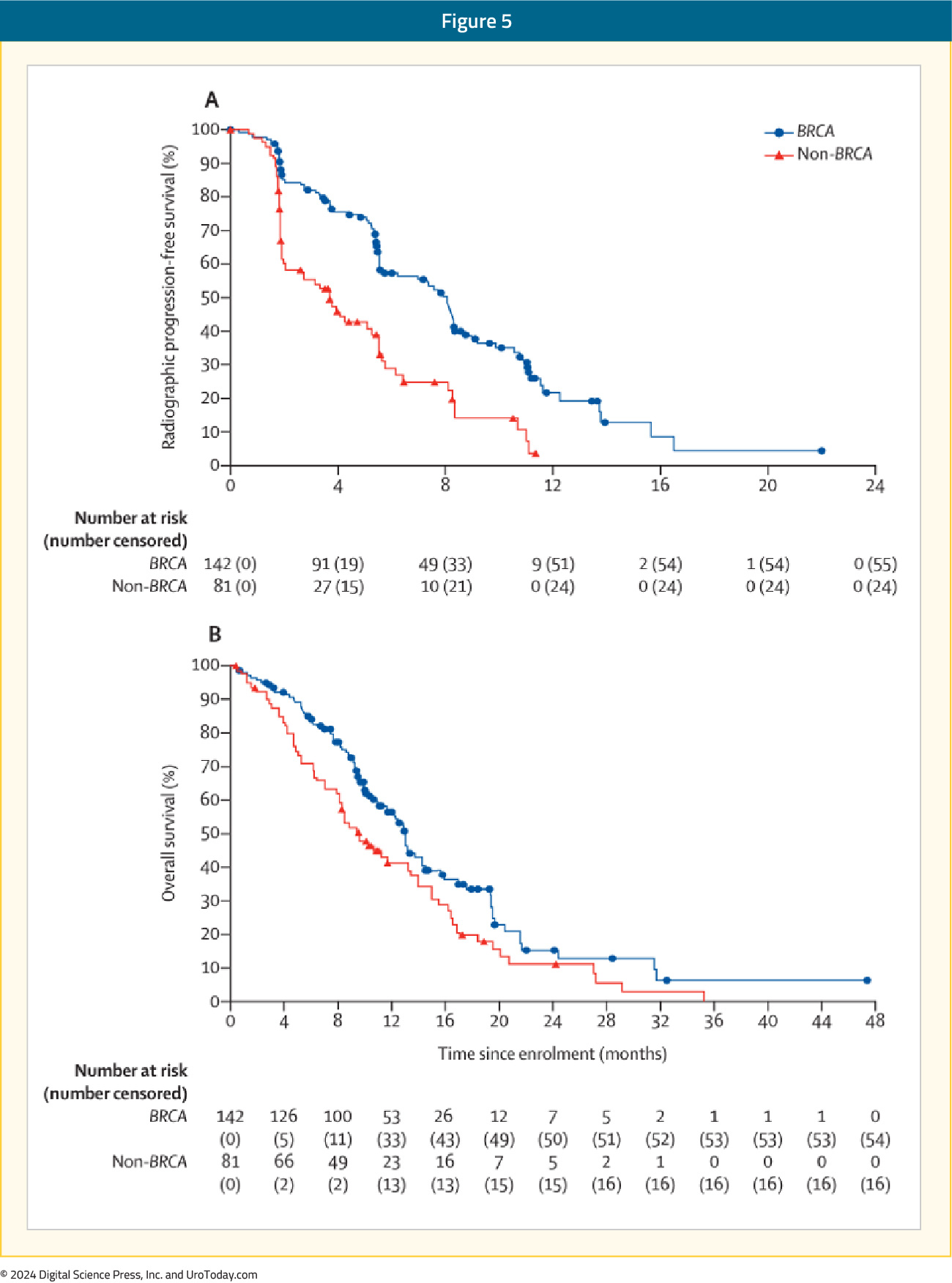
Management of Side Effects of PARP Inhibitors
The adverse event/safety profiles of all PARP inhibitors overlap considerably. The most common (any CTCAE grade) clinical side effects in phase III trials of rucaparib, olaparib and niraparib include:17
- Nausea: ~75%
- Fatigue: 60–70%
- Vomiting: ~35%
- Constipation: 20–40%
- Dysgeusia: 10–40%
- Anorexia: ~25%
- Abdominal pain: 25–30%
- Diarrhea: 20–30%
- Headache: 20–25%
- Cough: 10–15%
The most common (any CTCAE grade) lab abnormalities were:
- Anemia: 40–50%
- Thrombocytopenia: 15–60%
- Neutropenia: 20–30%
- Alanine aminotransferase (ALT) elevation: 5–36%
- Aspartate aminotransferase (AST) elevation: 2–28%
- Increased serum creatinine level: 10–15%
While nausea is the most common side effect of PARP inhibitor therapy, it tends to be mild in most cases. This side effect can be managed by taking the medication after a meal and an antiemetic (prochlorperazine or a 5-HT3 antagonist such as ondansetron) may be considered in patients who develop moderate or severe nausea and/or vomiting with PARP inhibitor therapy.
Close monitoring of patients following PARP inhibitor therapy initiation is required, particularly in the first three months, as hematologic adverse effects usually occur early, but not invariably, and regular blood counts should continue while patients are on treatment. Anemia is the most common hematologic toxicity observed with PARP inhibitors, with grade 3–4 anemia observed in 22% of patients on olaparib, 27% of patients on rucaparib, and 31% of patients on niraparib first-line maintenance therapy ovarian cancer trials.18-21 The management of such events may include dose reductions and/or interruptions, with transfusions reserved for symptomatic anemic events or if the hemoglobin level falls to <7 g/dL. Thrombocytopenia appears to be more common with niraparib at 61%, as opposed to olaparib (14%) or rucaparib (28%). The niraparib FDA label thus recommends obtaining weekly platelet levels during the first month of therapy.
Elevated serum creatinine level occurs within the first few weeks of therapy and is thought to be an on-target effect due to the inhibition of renal transporter proteins. Thus, serum creatinine-based estimation of renal function may be inaccurate in patients receiving PARP inhibitor therapy. Alternative methods of glomerular filtration rate (GFR) estimation such as radionuclide scan or serum-cystatin C must be used in cases where a more accurate GFR estimate is necessary. Elevation of AST and ALT also tends to typically occur within the first two cycles and can be transient. Treatment interruption may not be required for mild AST/ALT elevations, but serum bilirubin levels must be checked in all patients to evaluate for drug-induced liver injury.
Owing to their mechanism of action, there was a concern regarding treatment-emergent myelodysplastic syndrome (MDS) and acute myeloid leukemia (AML) with PARP inhibitor therapies. However, it appears that the risk of MDS/AML is <1.5%. Of the 2,351 patients treated in olaparib monotherapy trials, only 28 (<1.5%) developed MDS/AML. Of these, 25/28 patients had a BRCA mutation, two patients had a wild-type germline BRCA, and one patient had unknown BRCA mutation status. The duration of olaparib varied from < 6 months to > 2 years and all had received previous chemotherapy with platinum and/or other DNA damaging agents, or radiotherapy.17 If pancytopenia occurs at any point during PARP inhibitor therapy, treatment must be interrupted as per guidelines for the drug, and appropriate evaluation for MDS and AML must be undertaken. Therapy must be discontinued permanently if a diagnosis of MDS or AML is confirmed.
Another important consideration is the potential for clinically-significant drug-drug interactions (DDI) with all PARP inhibitors. Rucaparib and olaparib are primarily metabolized by different members of the cytochrome P450 enzyme family, resulting in only a partial overlap in DDIs. Niraparib is metabolized in the liver by carboxylesterase-catalyzed amide hydrolysis with cytochrome P450 playing only a negligible role.22 Many commonly used drugs (such as phenytoin, carbamazepine, ketoconazole, ciprofloxacin, digoxin) have uni- or bi-directional interactions with PARP inhibitors. Thus, careful attention must be paid to minimize DDI by avoiding, discontinuing, adjusting the dose, or clinical/lab monitoring of these medications before and during PARP therapy. Involving a dedicated oncology pharmacist, where available, may be a valuable aid in this treatment setting.
Conclusions and Future Directions
PARP inhibitor monotherapy has demonstrated promising outcomes for the treatment of HRR-mutated mCRPC patients with evidence of disease progression following treatment with an androgen receptor pathway inhibitor and/or taxane-based chemotherapy. As a result, there has been an increased interest in ‘moving up’ these agents along the disease spectrum, as well as combining PARP inhibitors with other agents that may have a synergistic mechanism of action.
Published March 2024
- Written by: Rashid K. Sayyid, MD, MSc Urologic Oncology Fellow University of Toronto Toronto, ON and Zachary Klaassen, MD, MSc Associate Professor Wellstar MCG Health Augusta, GA
- References:
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N Engl J Med. 2016;375:443-453.
- Xie T, Dickson K, Yee C, et al. Targeting Homologous Recombination Deficiency in Ovarian Cancer with PARP Inhibitors: Synthetic Lethal Strategies That Impact Overall Survival. Cancers (Basel). 2022;14(19):4621.
- FDA grants accelerated approval to rucaparib for BRCA-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-rucaparib-brca-mutated-metastatic-castration-resistant-prostate. Accessed on March 8, 2024.
- FDA approves olaparib for HRR gene-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-olaparib-hrr-gene-mutated-metastatic-castration-resistant-prostate-cancer. Accessed on March 8, 2024.
- FDA approves olaparib with abiraterone and prednisone (or prednisolone) for BRCA-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-olaparib-abiraterone-and-prednisone-or-prednisolone-brca-mutated-metastatic-castration. Accessed on March 8, 2024.
- FDA approves niraparib and abiraterone acetate plus prednisone for BRCA-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-niraparib-and-abiraterone-acetate-plus-prednisone-brca-mutated-metastatic-castration. Accessed on March 8, 2024.
- FDA approves talazoparib with enzalutamide for HRR gene-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-talazoparib-enzalutamide-hrr-gene-mutated-metastatic-castration-resistant-prostate. Accessed on March 8, 2024.
- Mateo J, Carreira S, Sandhu S, et al. DNA-repair defects and olaparib in metastatic prostate cancer. N Engl J Med. 2015;373:1697-1708.
- Mateo J, Porta N, Bianchini D, et al. Olaparib in patients with metastatic castration-resistant prostate cancer with DNA repair gene aberrations (TOPARP-B): a multicentre, open-label, randomised, phase 2 trial. Lancet Oncol. 2020;21(1):162-174.
- Hussain M, Mateo J, Fizazi K, et al. Survival with Olaparib in Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020;383:2345-2357.
- Abida W, Campbell D, Patnaik A, et al. Non-BRCA DNA Damage Repair Gene Alterations and Response to the PARP Inhibitor Rucaparib in Metastatic Castration-Resistant Prostate Cancer: Analysis From the Phase II TRITON2 Study. Clin Cancer Res. 2020;26(11):2487-2496.
- Abida W, Campbell D, Patnaik A, et al. Rucaparib for the Treatment of Metastatic Castration-resistant Prostate Cancer Associated with a DNA Damage Repair Gene Alteration: Final Results from the Phase 2 TRITON2 Study. Eur Urol 2023;84:321-330.
- Taza F, Holler AE, Fu W, et al. Differential Activity of PARP Inhibitors in BRCA1- Versus BRCA2-Altered Metastatic Castration-Resistant Prostate Cancer. JCO Precis Oncol 2021;5:PO.21.00070.
- Fizazi K, Piulats JM, Reaume MN, et al. Rucaparib or Physician’s Choice in Metastatic Prostate Cancer. N Engl J Med. 2023;388:719-732.
- de Bono JS, Mehra N, Scagliotti GV, et al. Talazoparib monotherapy in metastatic castration-resistant prostate cancer with DNA repair alterations (TALAPRO-1): an open-label, phase 2 trial. Lancet Oncol. 2021;22(9):1250-1264.
- Smith MR, Scher HI, Sandhu S, et al. Niraparib in patients with metastatic castration-resistant prostate cancer and DNA repair gene defects (GALAHAD): a multicentre, open-label, phase 2 trial. Lancet Oncol. 2022;23(3):362-373.
- LaFargue C, Dal Molin DZ, Sood AK, et al. Exploring and comparing adverse events between PARP inhibitors. Lancet Oncol. 2019;20(1):e15-e28.
- Gonzalez-Martin A, Pothuri B, Vergote I, et al: Niraparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2019;381:2391-2402.
- Moore K, Colombo N, Scambia G, et al: Maintenance olaparib in patients with newly diagnosed advanced ovarian cancer. N Engl J Med. 2018;379:2495-2505.
- Banerjee S, Moore KN, Colombo N, et al: Maintenance olaparib for patients with newly diagnosed advanced ovarian cancer and a BRCA mutation (SOLO1/GOG 3004): 5-Year follow-up of a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2021;22:1721-1731.
- Monk BJ, Parkinson C, Lim MC, et al: A randomized, phase III trial to evaluate rucaparib monotherapy as maintenance treatment in patients with newly diagnosed ovarian cancer (ATHENA-MONO/GOG-3020/ENGOT-ov45). J Clin Oncol. 2022;40:3952-3964.
- Sandhu SK, Schelman WR, Wilding G, et al. The poly (ADP-ribose) polymerase inhibitor niraparib (MK4827) in BRCA mutation carriers and patients with sporadic cancer: a phase 1 dose-escalation trial. Lancet Oncol. 2013;13(9):882-892.
The Current Landscape of Metastatic Castration-Resistant Prostate Cancer: Nearly Two Decades of Treatment Options
Prostate cancer, while commonly diagnosed as localized disease, remains the second leading cause of cancer mortality in the United States and Europe.1 For patients who die of prostate cancer, some will be initially diagnosed and treated for metastatic hormone-sensitive disease (mHSPC).
- Written by: Zachary Klaassen, MD MSc and Rashid Sayyid, MD MSc
- References:
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502-1512.
- Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996:14(6):1756-1764.
- Osoba D, Tannock IF, Ernst DS, Neville AJ. Health-related quality of life in men with metastatic prostate cancer treated with prednisone alone or mitoxantrone and prednisone. J Clin Oncol. 1999;17(6):1654-1663.
- de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet. 2010;376(9747):1147-1154.
- de Wit R, de Bono J, Sternberg CN, et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. N Engl J Med. 2019;381(26):2506-2518.
- Morgans AK, Hutson T, Guan AKD, et al. An economic evaluation of cabazitaxel versus a second androgen receptor-targeted agent (ARTA) for patients with metastatic castration-resistant prostate cancer previously treated with docetaxel and an ARTA: the United States payer perspective. BMC Health Serv Res. 2022;22(1):916.
Novel Treatment Targets in the Metastatic Castrate-Resistant Prostate Cancer Disease Space
Introduction
Since the United States Food and Drug Administration (FDA) approval of mitoxantrone in 19961 and docetaxel in 20042 for the treatment of patients with metastatic castrate-resistant prostate cancer, we have witnessed the approval of numerous additional agents/combinations in this disease space:
- Written by: Rashid K. Sayyid, MD MSc University of Toronto Toronto, ON & Zachary Klaassen, MD MSc Georgia Cancer Center Wellstar MCG Health Augusta, Georgia
- References:
- Tannock IF, Osoba D, Stockler MR, et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J Clin Oncol. 1996:14(6):1756-1764.
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351(15):1502-1512.
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med. 2010;363(5):411-422.
- de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet. 2010;376(9747):1147-1154.
- de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995-2005.
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368(2):138-148.
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187-1197.
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213-223.
- Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Engl J Med. 2014;371(5):424-433.
- Center for Drug Evaluation and Research. FDA grants accelerated approval to pembrolizumab for first tissue/site agnostic indication. U.S. Food and Drug Administration.
- Abida W, Patnaik A, Campbell D, et al. Rucaparib in Men with Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J Clin Oncol. 2020;38(32):3763-3772.
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020;382(22):2091-2102.
- Hofman MS, Emmett L, Sandhu S, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomized, open-label, phase 2 trial. Lancet. 2021;397(10276):797-804.
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021;385(12):1091-1103.
- Clarke N, Armstrong AJ, Thiery-Vuillemin A, et al. Abiraterone and olaparib for metastatic castration-resistant prostate cancer. NEJM Evidence. 2022.EVIDoa2200043.
- Agarwal N, Azad A, Carles J, et al. Talazoparib plus enzalutamide in men with first-line metastatic castration-resistant prostate cancer (TALAPRO-2): a randomised, placebo-controlled, phase 3 trial. The Lancet. 2023;402(10398):291-303.
- George DJ, Sartor O, Miller K, et al. Treatment Patterns and Outcomes in Patients With Metastatic Castration-resistant Prostate Cancer in a Real-world Clinical Practice Setting in the United States. Clin Genitourin Cancer. 2020;18(4):284-94.
- Koivisto P, Kononen J, Palmberg C, et al. Androgen Receptor Gene Amplification: A Possible Molecular Mechanism for Androgen Deprivation Therapy Failure in Prostate Cancer. Cancer Res. 1997;57(2):314-9.
- Henzler C, Li Y, Yang R, et al. Truncation and constitutive activation of the androgen receptor by diverse genomic rearrangements in prostate cancer. Nat Commun. 2016;7:13668.
- Pachynski RK, Iannotti N, Laccetti AL, et al. Oral EPI-7386 in patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2023;41(Suppl 6):177.
- Laccetti AL, Chatta GS, Iannotti N, et al. Phase 1/2 study of EPI-7386 in combination with enzalutamide (enz) compared with enz alone in subjects with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2023;41(Suppl 6):179.
- Desai K, Serritella AV, Stadler WM, et al. Phase I trial of enzalutamide (Enz) plus the glucocorticoid receptor antagonist relacorilant (Rela) for patients with metastatic castration resistant prostate cancer. J Clin Oncol. 2023;41(Suppl 6):5062.
- Fizazi K, Cook N, Barthelemy P, et al. Phase 1 results of the ODM-208 first-in-human phase 1-2 trial in patients with metastatic castration-resistant prostate cancer (CYPIDES). J Clin Oncol. 2022;40(Suppl 6):18.
- Smith MR, Agarwal N, Todenhofer T, et al. CYCLONE 2: A phase 2/3, randomized, placebo-controlled study of abiraterone acetate plus prednisone with or without abemaciclib in patients with metastatic castration-resistant prostate cancer. J Clin Oncol. 2022;40(Suppl 6):198.
- Dorff TB, Blanchard S, Martirosyan H, et al. Final results from phase I study of PSCA-targeted chimeric antigen receptor (CAR) T cells in patients with metastatic castration resistant prostate cancer (mCRPC). J Clin Oncol. 2023;41(Suppl 6):5019.
- Sandhu S, Joshua AM, Emmett L, et al. LuPARP: Phase 1 trial of 177Lu-PSMA-617 and olaparib in patients with metastatic castration resistant prostate cancer (mCRPC). J Clin Oncol. 2023;41(Suppl 6):5005.
- Kostos LK, Buteau JP, Kong G, et al. LuCAB: A phase I/II trial evaluating cabazitaxel in combination with [177Lu]Lu-PSMA-617 in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol. 2023;41(Suppl 6):TPS278.
- Teiluf K, Seidl C, Blechert B, et al. α-Radioimmunotherapy with 213Bi-anti-CD38 immunoconjugates is effective in a mouse model of human multiple myeloma. Oncotarget. 2015;6:4692-4703.
- Ma J, Li L, Liao T, Fong W, Zhang C. Efficacy and Safety of 225Ac-PSMA-617-Targeted Alpha Therapy in Metastatic Castration-Resistant Prostate Cancer: A Systematic Review and Meta-Analysis. Front Oncol. 2022;12:796657.
- Nauseef JT, Sun MP, Thomas C, et al. A phase I/II dose-escalation study of fractionated 225Ac-J591 for progressive metastatic castration-resistant prostate cancer (mCRPC) in patients with prior treatment with 177Lu-PSMA. J Clin Oncol. 2023;41(Supp 6):TPS288.
The Role of Remote Interactions in Genitourinary Oncology: Implications for Practice Change in Light of the COVID-19 Pandemic
- Written by: Zachary Klaassen, MD, MSc
- References:
- Guan WJ, Ni ZY, Hu Y, et al. Clinical Characteristics of Coronavirus Disease 2019 in China. The New England journal of medicine. 2020.
- Dai M, Liu D, Liu M, et al. Patients with cancer appear more vulnerable to SARS-COV-2: a multi-center study during the COVID-19 outbreak. Cancer Discov. 2020.
- Team CC-R. Severe Outcomes Among Patients with Coronavirus Disease 2019 (COVID-19) - United States, February 12-March 16, 2020. MMWR Morb Mortal Wkly Rep. 2020;69(12):343-346.
- Grasselli G, Zangrillo A, Zanella A, et al. Baseline Characteristics and Outcomes of 1591 Patients Infected With SARS-CoV-2 Admitted to ICUs of the Lombardy Region, Italy. JAMA : the journal of the American Medical Association. 2020.
- Myers LC, Parodi SM, Escobar GJ, Liu VX. Characteristics of Hospitalized Adults With COVID-19 in an Integrated Health Care System in California. JAMA : the journal of the American Medical Association. 2020.
- Richardson S, Hirsch JS, Narasimhan M, et al. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA : the journal of the American Medical Association. 2020.
- Boehm K, Ziewers S, Brandt MP, et al. Telemedicine Online Visits in Urology During the COVID-19 Pandemic-Potential, Risk Factors, and Patients' Perspective. European urology. 2020;78(1):16-20.
- Castaneda P, Ellimoottil C. Current use of telehealth in urology: a review. World journal of urology. 2019.
- Medicaid CfM. MEDICARE TELEMEDICINE HEALTH CARE PROVIDER FACT SHEET. 2020; https://www.cms.gov/newsroom/fact-sheets/medicare-telemedicine-health-care-provider-fact-sheet. Accessed June 1, 2020.
- Schaffert R, Dahinden U, Hess T, et al. [Evaluation of a prostate cancer Ehealth tutorial : Development and testing of the website prostata-information.ch]. Urologe A. 2018;57(2):164-171.
- Berry DL, Hong F, Blonquist TM, et al. Decision Support with the Personal Patient Profile-Prostate: A Multicenter Randomized Trial. The Journal of urology. 2018;199(1):89-97.
- Parsons JK, Zahrieh D, Mohler JL, et al. Effect of a Behavioral Intervention to Increase Vegetable Consumption on Cancer Progression Among Men With Early-Stage Prostate Cancer: The MEAL Randomized Clinical Trial. JAMA : the journal of the American Medical Association. 2020;323(2):140-148.
- Skolarus TA, Metreger T, Wittmann D, et al. Self-Management in Long-Term Prostate Cancer Survivors: A Randomized, Controlled Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2019;37(15):1326-1335.
- Viers BR, Lightner DJ, Rivera ME, et al. Efficiency, satisfaction, and costs for remote video visits following radical prostatectomy: a randomized controlled trial. European urology. 2015;68(4):729-735.
- Leahy M, Krishnasamy M, Herschtal A, et al. Satisfaction with nurse-led telephone follow up for low to intermediate risk prostate cancer patients treated with radical radiotherapy. A comparative study. Eur J Oncol Nurs. 2013;17(2):162-169.
- Belarmino A, Walsh R, Alshak M, Patel N, Wu R, Hu JC. Feasibility of a Mobile Health Application To Monitor Recovery and Patient-reported Outcomes after Robot-assisted Radical Prostatectomy. Eur Urol Oncol. 2019;2(4):425-428.
- Lange L, Fink J, Bleich C, Graefen M, Schulz H. Effectiveness, acceptance and satisfaction of guided chat groups in psychosocial aftercare for outpatients with prostate cancer after prostatectomy. Internet Interv. 2017;9:57-64.
- Trinh L, Arbour-Nicitopoulos KP, Sabiston CM, et al. RiseTx: testing the feasibility of a web application for reducing sedentary behavior among prostate cancer survivors receiving androgen deprivation therapy. Int J Behav Nutr Phys Act. 2018;15(1):49.
- Lee BJ, Park YH, Lee JY, Kim SJ, Jang Y, Lee JI. Smartphone Application Versus Pedometer to Promote Physical Activity in Prostate Cancer Patients. Telemed J E Health. 2019;25(12):1231-1236.
- Novara G, Checcucci E, Crestani A, et al. Telehealth in Urology: A Systematic Review of the Literature. How Much Can Telemedicine Be Useful During and After the COVID-19 Pandemic? European Urology (in press). 2020.
- Kontopantelis E, Roland M, Reeves D. Patient experience of access to primary care: identification of predictors in a national patient survey. BMC Fam Pract. 2010;11:61.
- Kane LT, Thakar O, Jamgochian G, et al. The role of telehealth as a platform for postoperative visits following rotator cuff repair: a prospective, randomized controlled trial. J Shoulder Elbow Surg. 2020;29(4):775-783.
- Wallis CJD, Morton G, Herschorn S, et al. The effect of selection and referral biases for the treatment of localised prostate cancer with surgery or radiation. British journal of cancer. 2018;118(10):1399-1405.
- Meti N, Rossos PG, Cheung MC, Singh S. Virtual Cancer Care During and Beyond the COVID-19 Pandemic: We Need to Get It Right. JCO Oncol Pract. 2020:OP2000281.
- Holstead RG, Robinson AG. Discussing Serious News Remotely: Navigating Difficult Conversations During a Pandemic. JCO Oncol Pract. 2020:OP2000269.
- Roberts ET, Mehrotra A. Assessment of Disparities in Digital Access Among Medicare Beneficiaries and Implications for Telemedicine. JAMA internal medicine. 2020.
- Lam K, Lu AD, Shi Y, Covinsky KE. Assessing Telemedicine Unreadiness Among Older Adults in the United States During the COVID-19 Pandemic. JAMA internal medicine. 2020.
- de la Torre-Diez I, Lopez-Coronado M, Vaca C, Aguado JS, de Castro C. Cost-utility and cost-effectiveness studies of telemedicine, electronic, and mobile health systems in the literature: a systematic review. Telemed J E Health. 2015;21(2):81-85.
- Jiang X, Ming WK, You JH. The Cost-Effectiveness of Digital Health Interventions on the Management of Cardiovascular Diseases: Systematic Review. J Med Internet Res. 2019;21(6):e13166.
- Shepperd S, Iliffe S. Hospital at home versus in-patient hospital care. Cochrane Database Syst Rev. 2001(3):CD000356.
- Qaddoura A, Yazdan-Ashoori P, Kabali C, et al. Efficacy of Hospital at Home in Patients with Heart Failure: A Systematic Review and Meta-Analysis. PloS one. 2015;10(6):e0129282.
- Caplan GA, Coconis J, Woods J. Effect of hospital in the home treatment on physical and cognitive function: a randomized controlled trial. The journals of gerontology Series A, Biological sciences and medical sciences. 2005;60(8):1035-1038.
- Raphael R, Yves D, Giselle C, Magali M, Odile CM. Cancer treatment at home or in the hospital: what are the costs for French public health insurance? Findings of a comprehensive-cancer centre. Health Policy. 2005;72(2):141-148.
- K. SR, Magee D, Hird AE, et al. Reoperation within 30 Days of Radical Cystectomy: Identifying High-Risk Patients and Complications Using ACS-NSQIP Database. Can Urol Assoc J (in press). 2020.
- Metcalf M, Glazyrine V, Glavin K, et al. The Feasibility of a Health Care Application in the Treatment of Patients Undergoing Radical Cystectomy. The Journal of urology. 2019;201(5):902-908.
- Catto JWF, Khetrapal P, Ambler G, et al. Multidomain Quantitative Recovery Following Radical Cystectomy for Patients Within the Robot-assisted Radical Cystectomy with Intracorporeal Urinary Diversion Versus Open Radical Cystectomy Randomised Controlled Trial: The First 30 Patients. European urology. 2018;74(4):531-534.
- van Hout L, Bokkerink WJV, Ibelings MS, Vriens P. Perioperative monitoring of inguinal hernia patients with a smartphone application. Hernia. 2020;24(1):179-185.
- Raja JM, Elsakr C, Roman S, et al. Apple Watch, Wearables, and Heart Rhythm: where do we stand? Ann Transl Med. 2019;7(17):417.
- Krishnan N, Li B, Jacobs BL, et al. The Fate of Radical Cystectomy Patients after Hospital Discharge: Understanding the Black Box of the Pre-readmission Interval. Eur Urol Focus. 2018;4(5):711-717.
- Krishnan N, Liu X, Lavieri MS, et al. A Model to Optimize Followup Care and Reduce Hospital Readmissions after Radical Cystectomy. The Journal of urology. 2016;195(5):1362-1367.
- Cai S, Grubbs A, Makineni R, Kinosian B, Phibbs CS, Intrator O. Evaluation of the Cincinnati Veterans Affairs Medical Center Hospital-in-Home Program. J Am Geriatr Soc. 2018;66(7):1392-1398.
- Richards SH, Coast J, Gunnell DJ, Peters TJ, Pounsford J, Darlow MA. Randomised controlled trial comparing effectiveness and acceptability of an early discharge, hospital at home scheme with acute hospital care. Bmj. 1998;316(7147):1796-1801.
- Ramkumar PN, Haeberle HS, Ramanathan D, et al. Remote Patient Monitoring Using Mobile Health for Total Knee Arthroplasty: Validation of a Wearable and Machine Learning-Based Surveillance Platform. J Arthroplasty. 2019;34(10):2253-2259.
- Breteler MJM, KleinJan E, Numan L, et al. Are current wireless monitoring systems capable of detecting adverse events in high-risk surgical patients? A descriptive study. Injury. 2019.
- Soukup T, Lamb BW, Arora S, Darzi A, Sevdalis N, Green JS. Successful strategies in implementing a multidisciplinary team working in the care of patients with cancer: an overview and synthesis of the available literature. J Multidiscip Healthc. 2018;11:49-61.
- Specchia ML, Frisicale EM, Carini E, et al. The impact of tumor board on cancer care: evidence from an umbrella review. BMC Health Serv Res. 2020;20(1):73.
- Charara RN, Kreidieh FY, Farhat RA, et al. Practice and Impact of Multidisciplinary Tumor Boards on Patient Management: A Prospective Study. J Glob Oncol. 2017;3(3):242-249.
- Salami AC, Barden GM, Castillo DL, et al. Establishment of a Regional Virtual Tumor Board Program to Improve the Process of Care for Patients With Hepatocellular Carcinoma. J Oncol Pract. 2015;11(1):e66-74.
- Lesslie M, Parikh JR. Implementing a Multidisciplinary Tumor Board in the Community Practice Setting. Diagnostics (Basel). 2017;7(4).
- McGeady JB, Blaschko SD, Brajtbord JS, Sewell JL, Chen AH, Breyer BN. Electronic Preconsultation as a Method of Quality Improvement for Urological Referrals. Urology Practice 2014;1:172-175.
- Chertack N, Lotan Y, Mayorga C, Mauck R. Implementation of a Urology E-Consult Service at a Safety Net County Hospital. Urology Practice.
- Witherspoon L, Liddy C, Afkham A, Keely E, Mahoney J. Improving access to urologists through an electronic consultation service. Can Urol Assoc J. 2017;11(8):270-274.
- Vimalananda VG, Gupte G, Seraj SM, et al. Electronic consultations (e-consults) to improve access to specialty care: a systematic review and narrative synthesis. J Telemed Telecare. 2015;21(6):323-330.
- Rosner BI, Gottlieb M, Anderson WN. Effectiveness of an Automated Digital Remote Guidance and Telemonitoring Platform on Costs, Readmissions, and Complications After Hip and Knee Arthroplasties. J Arthroplasty. 2018;33(4):988-996 e984.
- Balakrishnan AS, Nguyen HG, Shinohara K, Au Yeung R, Carroll PR, Odisho AY. A Mobile Health Intervention for Prostate Biopsy Patients Reduces Appointment Cancellations: Cohort Study. J Med Internet Res. 2019;21(6):e14094.
- Asch DA, Nicholson S, Berger ML. Toward Facilitated Self-Service in Health Care. The New England journal of medicine. 2019;380(20):1891-1893.
The Current State of Treatment Implementation for mCRPC in North America
Introduction
There have been significant advances in the metastatic castrate-resistant prostate cancer (mCRPC) treatment landscape with the emergence and approval of numerous agents in this disease space.- Written by: Rashid Sayyid, MD MSc University of Toronto Toronto, ON & Zachary Klaassen, MD MSc Georgia Cancer Center Wellstar MCG Health Augusta, GA
- References:
- Freedland SJ, Davis M, Epstein AJ, et al. Real-world treatment patterns and overall survival among men with Metastatic Castration-Resistant Prostate Cancer (mCRPC) in the US Medicare population. Prostate Cancer Prostatic Dis. 2023.
- FDA grants accelerated approval to rucaparib for BRCA-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-grants-accelerated-approval-rucaparib-brca-mutated-metastatic-castration-resistant-prostate. Accessed on October 29, 2023.
- FDA approves olaparib for HRR gene-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-olaparib-hrr-gene-mutated-metastatic-castration-resistant-prostate-cancer. Accessed on October 29, 2023.
- FDA D.I.S.C.O. Burst Edition: FDA approval of Lynparza (olaparib), with abiraterone and prednisone, for BRCA-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approval-lynparza-olaparib-abiraterone-and-prednisone-brca-mutated#:~:text=On%20May%2031%2C%202023%2C%20the,FDA%2Dapproved%20companion%20diagnostic%20test.. Accessed on October 29, 2023.
- FDA approves talazoparib with enzalutamide for HRR gene-mutated metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/drug-approvals-and-databases/fda-approves-talazoparib-enzalutamide-hrr-gene-mutated-metastatic-castration-resistant-prostate. Accessed on October 29, 2023.
- FDA approves Pluvicto for metastatic castration-resistant prostate cancer. https://www.fda.gov/drugs/resources-information-approved-drugs/fda-approves-pluvicto-metastatic-castration-resistant-prostate-cancer. Accessed on October 29, 2023.
- Swami U, Aggarwal H, Zhou M, et al. Treatment Patterns, Clinical Outcomes, Health Care Resource Utilization and Costs in Older Patients With Metastatic Castration-Resistant Prostate Cancer in the United States: An Analysis of SEER-Medicare Data. Clin Genitourin Cancer. 2023;21(5):517-529.
- Shayegan B, Wallis CJD, Malone S, et al. Real-world use of systemic therapies in men with metastatic castration resistant prostate cancer (mCRPC) in Canada. Urol Oncol. 2022;40(5):192.e1-192.e9.
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med. 2004;351:1502-1512.
- Khalaf DJ, Annala M, Taavitsainen S, et al. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label, phase 2, crossover trial. Lancet Oncol. 2019;20(12):1730-1739.
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2020;382(22):2091-2102.
- Fizazi K, Piulats JM, Reaume MN, et al. Rucaparib or Physician’s Choice in Metastatic Prostate Cancer. N Engl J Med. 2023;388:719-732.
- de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364(21):1995-2005.
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Engl J Med. 2012;367:1187-1197.
- de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: A randomised open-label trial. Lancet. 2010;376(9747):1147-1154.
- Sartor O, de Bono J, Chi KN et al. Lutetium-177-PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021;385(12):1091-1103.
- Hofman MS, Emmett L, Sandhu S, et al. [(177)Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): A randomized, open-label, phase 2 trial. Lancet. 2021;397(10276):797-804.
- de Wit R, de Bono J, Sternberg CN, et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. N Engl J Med. 2019;381(26):2506-2518.
PARP Inhibitors in Prostate Cancer
- Written by: Zachary Klaassen, MD, MSc
- References:
- Rubin MA, Maher CA, Chinnaiyan AM. Common gene rearrangements in prostate cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2011;29(27):3659-3668.
- Kunkel TA, Erie DA. DNA mismatch repair. Annu Rev Biochem. 2005;74:681-710.
- Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. The New England journal of medicine. 2016;375(5):443-453.
- Castro E, Romero-Laorden N, Del Pozo A, et al. PROREPAIR-B: A Prospective Cohort Study of the Impact of Germline DNA Repair Mutations on the Outcomes of Patients With Metastatic Castration-Resistant Prostate Cancer. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2019;37(6):490-503.
- Nicolosi P, Ledet E, Yang S, et al. Prevalence of Germline Variants in Prostate Cancer and Implications for Current Genetic Testing Guidelines. JAMA Oncol. 2019;5(4):523-528.
- Dantzer F, de La Rubia G, Menissier-De Murcia J, Hostomsky Z, de Murcia G, Schreiber V. Base excision repair is impaired in mammalian cells lacking Poly(ADP-ribose) polymerase-1. Biochemistry. 2000;39(25):7559-7569.
- McCabe N, Turner NC, Lord CJ, et al. Deficiency in the repair of DNA damage by homologous recombination and sensitivity to poly(ADP-ribose) polymerase inhibition. Cancer Res. 2006;66(16):8109-8115.
- Gudmundsdottir K, Ashworth A. The roles of BRCA1 and BRCA2 and associated proteins in the maintenance of genomic stability. Oncogene. 2006;25(43):5864-5874.
- Farmer H, McCabe N, Lord CJ, et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature. 2005;434(7035):917-921.
- Ashworth A. A synthetic lethal therapeutic approach: poly(ADP) ribose polymerase inhibitors for the treatment of cancers deficient in DNA double-strand break repair. J Clin Oncol. 2008;26(22):3785-3790.
- Fong PC, Boss DS, Yap TA, et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N Engl J Med. 2009;361(2):123-134.
- Mateo J, Carreira S, Sandhu S, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. The New England journal of medicine. 2015;373(18):1697-1708.
- Mateo J, Porta N, McGovern U, et al. TOPARP-B: A phase II randomized trial of the poly(ADP)-ribose polymerase (PARP) inhibitor olaparib for metastatic castration resistant prostate cancers (mCRPC) with DNA damage repair (DDR) alterations. J Clin Oncol. 2019;37(15_suppl):5005.
- de Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. The New England journal of medicine. 2020.
- de Wit R, de Bono J, Sternberg CN, et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. The New England journal of medicine. 2019;381(26):2506-2518.
- Clarke N, Wiechno P, Alekseev B, et al. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: a randomised, double-blind, placebo-controlled, phase 2 trial. The lancet oncology. 2018;19(7):975-986.
- Abida W, Bryce AH, Vogelzang N, et al. Preliminary Results From TRITON2: A Phase II Study of Rucaparib in Patients with mCRPC Associated with Homologous Recombination Repair Gene Alterations. Ann Oncol. 2018;29(suppl_8):viii271.
- Smith MR, Sandhu S, Kelly WK, et al. Phase II study of niraparib in patients with metastatic castration-resistant prostate cancer (mCRPC) and biallelic DNA-repair gene defects (DRD): Preliminary results of GALAHAD. J Clin Oncol. 2019;37(7_suppl):202.
- Hussain M, Daignault-Newton S, Twardowski PW, et al. Targeting Androgen Receptor and DNA Repair in Metastatic Castration-Resistant Prostate Cancer: Results From NCI 9012. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2018;36(10):991-999.
- Hussain M, Carducci MA, Slovin S, et al. Targeting DNA repair with combination veliparib (ABT-888) and temozolomide in patients with metastatic castration-resistant prostate cancer. Invest New Drugs. 2014;32(5):904-912.
- Yu EY, Massard C, Retz M, et al. Keynote-365 cohort a: Pembrolizumab (pembro) plus olaparib in docetaxel-pretreated patients (pts) with metastatic castrate-resistant prostate cancer (mCRPC). J Clin Oncol. 2019;37(7_suppl):145.
PARP Inhibitor Plus Androgen Receptor Signaling Inhibitor Combinations: Will This Be The Future of mCRPC First-Line Therapy?
Introduction: Despite the approval of numerous agents in this setting, patients with metastatic castrate-resistant prostate cancer (mCRPC) have a poor prognosis, with an estimated median overall survival (OS) of approximately three years with currently approved first-line agents.1-3
- Written by: Rashid K. Sayyid, MD MSc & Zachary Klaassen, MD MSc
- References:
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med 2013; 368(2):138-48.
- Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in Men with Chemotherapy-naïve Metastatic Castration-resistant Prostate Cancer: Extended Analysis of the Phase 3 PREVAIL Study. Eur Urol 2017; 71(2):1 51-4.
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Engl J Med 2004; 351(15):1502-12.
- George DJ, Sartor O, Miller K, et al. Treatment Patterns and Outcomes in Patients With Metastatic Castration-resistant Prostate Cancer in a Real-world Clinical Practice Setting in the United States. Clin Genitourin Cancer 2020; 18(4):284-94.
- De Bono J, Mateo J, Fizazi K, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med 2020; 382(22): 2091-102.
- Abida W, Patnaik A, Campbell D, et al. Rucaparib in Men With Metastatic Castration-Resistant Prostate Cancer Harboring a BRCA1 or BRCA2 Gene Alteration. J Clin Oncol 2020; 38(32): 3763-72.
- Fizazi K, Piulats JM, Reaume MN, et al. Rucaparib or Physician’s Choice in Metastatic Prostate Cancer. N Engl J Med 2023; 388: 719-32.
- Asim M, Tarish F, Zecchini HI, et al. Synthetic lethality between androgen receptor signalling and the PARP pathway in prostate cancer. Nat Commun 2017; 8: 374.
- Schiewer MJ, Goodwin JF, Han S, et al. Dual roles of PARP-1 promote cancer growth and progression. Cancer Discov 2012; 2: 1134-49.
- Li L, Karanika S, Yang G, et al. Androgen receptor inhibitor-induced “BRCAness” and PARP inhibition are synthetically lethal for castration-resistant prostate cancer. Sci Signal 2017; 10: eaam7479.
- Clarke N, Wiechno P, Alekseev B, et al. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol 2018; 19: 975-86.
- Clarke NW, Armstrong AJ, Thiery-Vuillemin A, et al. Abiraterone and Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med 2022; 1(9).
- Clarke NW, Armstrong AJ, Thiery-Vuillemin A, et al. Final overall survival (OS) in PROpel: abiraterone (abi) and olaparib (ola) versus abiraterone and placebo (pbo) as first-line (1L) therapy for metastatic castration-resistant prostate cancer (mCRPC). J Clin Oncol 2023; 41(Suppl 6; abstr LBA16).
- Chi KN, Rathkopf DE, Smith MR, et al. Niraparib and Abiraterone Acetate for Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol 2023; JCO2201649.
- Agarwal N, Azad A, Carles J, et al. Abiraterone and Olaparib for Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol 2023; 41 (Suppl 6; abstr LBA17).
The Impact of COVID-19 on Oncology Clinical Trials
Since the beginning of the COVID-19 pandemic in early 2020, the diagnosis, treatment and surveillance of cancer has been transformed globally. The heavy demand for resources, exacerbated by limited excess health system capacity, means that health care systems have become quickly overwhelmed and hospitals have become sources for virus transmission.
- Written by: Zachary Klaassen, MD, MSc
- References:
1. COVID, CDC, and Response Team. "Severe outcomes among patients with coronavirus disease 2019 (COVID-19)—United States, February 12–March 16, 2020." MMWR Morb Mortal Wkly Rep 69, no. 12 (2020): 343-346.
2. Thornton, Jacqui. "Clinical trials suspended in UK to prioritise covid-19 studies and free up staff." BMJ 368 (2020): m1172.
3. Majumdar, Sumit R., Matthew T. Roe, Eric D. Peterson, Anita Y. Chen, W. Brian Gibler, and Paul W. Armstrong. "Better outcomes for patients treated at hospitals that participate in clinical trials." Archives of internal medicine 168, no. 6 (2008): 657-662.
4. Skrutkowska, Myriam, and Charles Weijer. "Do patients with breast cancer participating in clinical trials receive better nursing care?." In Oncology nursing forum, vol. 24, no. 8, pp. 1411-1416. 1997.
5. McDermott, Mary M., and Anne B. Newman. "Preserving clinical trial integrity during the coronavirus pandemic." Jama (2020).
6. Marandino, Laura, Massimo Di Maio, Giuseppe Procopio, Saverio Cinieri, Giordano Domenico Beretta, and Andrea Necchi. "The Shifting Landscape of Genitourinary Oncology During the COVID-19 Pandemic and how Italian Oncologists Reacted: Results from a National Survey." European Urology (2020).
7. Wallis, Christopher JD, Giacomo Novara, Laura Marandino, Axel Bex, Ashish M. Kamat, R. Jeffrey Karnes, Todd M. Morgan et al. "Risks from Deferring Treatment for Genitourinary Cancers: A Collaborative Review to Aid Triage and Management During the COVID-19 Pandemic." European Urology (2020).
8. Segelov, Eva, Hans Prenen, Daphne Day, C. Raina Macintyre, Estelle Mei Jye Foo, Raghib Ali, Quanyi Wang et al. "Impact of the COVID-19 Epidemic on a Pan-Asian Academic Oncology Clinical Trial." JCO global oncology 6 (2020): 585.
9. Wang, Hongkai, Junlong Wu, Yu Wei, Yao Zhu, and Dingwei Ye. "Surgical Volume, Safety, Drug Administration, and Clinical Trials During COVID-19: Single-center Experience in Shanghai, China." European Urology (2020).
10. Waterhouse D, Harvey RD, Hurley P, Levit LA, Klepin HD. "Early Impact of COVID-19 on the Conduct of Oncology Clinical Trials and Long-term Opportunities for Transformation: Findings from an American Society of Clinical Oncology Survey." JCO Oncology Practice. 2020.
11. US Food and Drug Administration. "FDA guidance on conduct of clinical trials of medical products during COVID-19 pandemic: guidance for industry, investigators, and institutional review boards." (2020).
12. Tan, Aaron C., David M. Ashley, and Mustafa Khasraw. "Adapting to a pandemic-conducting oncology trials during the SARS-CoV-2 pandemic." Clinical Cancer Research (2020).
13. Khozin, Sean, and Andrea Coravos. "Decentralized Trials in the Age of Real-World Evidence and Inclusivity in Clinical Investigations." Clinical pharmacology and therapeutics 106, no. 1 (2019): 25-27.
14. Galsky, Matthew D., Mohamed Shahin, Rachel Jia, David R. Shaffer, Kiev Gimpel-Tetra, Che-Kai Tsao, Charles Baker et al. "Telemedicine-enabled clinical trial of metformin in patients with prostate cancer." JCO clinical cancer informatics 1 (2017): 1-10.
15. Borno, Hala T., and Eric J. Small. "Does the COVID-19 outbreak identify a broader need for an urgent transformation of cancer clinical trials research?." Contemporary Clinical Trials 92 (2020).
16. Duley, Lelia, Karen Antman, Joseph Arena, Alvaro Avezum, Mel Blumenthal, Jackie Bosch, Sue Chrolavicius et al. "Specific barriers to the conduct of randomized trials." Clinical Trials 5, no. 1 (2008): 40-48.
17. Uren, Shannon C., Mitchell B. Kirkman, Brad S. Dalton, and John R. Zalcberg. "Reducing clinical trial monitoring resource allocation and costs through remote access to electronic medical records." Journal of oncology practice 9, no. 1 (2013): e13-e16.
Artificial Intelligence and Prostate Cancer: Risk Stratification After Primary Therapy, ADT Treatment Intensification, and Evaluation of Metastatic Disease
Artificial intelligence continues to transform the field of medicine, including the management of prostate cancer. In this Center of Excellence article, we discuss the contemporary literature evaluating artificial intelligence for risk stratification after primary therapy, ADT treatment intensification, and evaluation of metastatic disease.
- Written by: Zachary Klaassen, MD, MSc and Rashid K. Sayyid, MD, MSc
- References:
- Tan YG, Fang AHS, Lim JKS, et al. Incorporating artificial intelligence in urology: Supervised machine learning algorithms demonstrate comparative advantage over nomograms in predicting biochemical recurrence after prostatectomy. Prostate. 2022;82: 298-305.
- McIntosh C, Conroy L, Tjong MC, et al. Clinical integration of machine learning for curative-intent radiation treatment of patients with prostate cancer. Nat Med. 2021;27: 999-1005.
- Esteva A, Feng J, van der Wal D, et al. Prostate cancer therapy personalization via multi-modal deep learning on randomized phase III clinical trials. NPJ Digit Med. 2022;5: 71.
- Yang R, Zhu D, Howard LE, et al. Identification of Patients With Metastatic Prostate Cancer With Natural Language Processing and Machine Learning. JCO Clin Cancer Inform. 2022;6: e2100071.
- Elledge CR, LaVigne AW, Fiksel J, et al. External Validation of the Bone Metastases Ensemble Trees for Survival (BMETS) Machine Learning Model to Predict Survival in Patients With Symptomatic Bone Metastases. JCO Clin Cancer Inform. 2021;5: 304-314.
- Kartasalo K, Bulten W, Delahunt B, et al. Artificial Intelligence for Diagnosis and Gleason Grading of Prostate Cancer in Biopsies-Current Status and Next Steps. Eur Urol Focus. 2021;7: 687-691.
Androgen Receptor Signaling in Castration-Resistant Prostate Cancer
- Written by: Zachary Klaassen, MD, MSc
- References:
1. Huggins, Charles, and Clarence V. Hodges. "Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate." The Journal of urology 167, no. 2 Part 2 (2002): 948-951.
2. Coutinho, Isabel, Tanya K. Day, Wayne D. Tilley, and Luke A. Selth. "Androgen receptor signaling in castration-resistant prostate cancer: a lesson in persistence." Endocrine-related cancer 23, no. 12 (2016): T179-T197.
3. Visakorpi, Tapio, Eija Hyytinen, Pasi Koivisto, Minna Tanner, Riitta Keinänen, Christian Palmberg, Aarno Palotie, Teuvo Tammela, Jorma Isola, and Olli-P. Kallioniemi. "In vivo amplification of the androgen receptor gene and progression of human prostate cancer." Nature genetics 9, no. 4 (1995): 401-406.
4. Chen, Charlie D., Derek S. Welsbie, Chris Tran, Sung Hee Baek, Randy Chen, Robert Vessella, Michael G. Rosenfeld, and Charles L. Sawyers. "Molecular determinants of resistance to antiandrogen therapy." Nature medicine 10, no. 1 (2004): 33-39.
5. Wyatt, Alexander W., and Martin E. Gleave. "Targeting the adaptive molecular landscape of castration‐resistant prostate cancer." EMBO molecular medicine 7, no. 7 (2015): 878-894.
6. Robinson, Dan, Eliezer M. Van Allen, Yi-Mi Wu, Nikolaus Schultz, Robert J. Lonigro, Juan-Miguel Mosquera, Bruce Montgomery et al. "Integrative clinical genomics of advanced prostate cancer." Cell 161, no. 5 (2015): 1215-1228.
7. Grasso, Catherine S., Yi-Mi Wu, Dan R. Robinson, Xuhong Cao, Saravana M. Dhanasekaran, Amjad P. Khan, Michael J. Quist et al. "The mutational landscape of lethal castration-resistant prostate cancer." Nature 487, no. 7406 (2012): 239-243.
8. Cai, Changmeng, Housheng Hansen He, Sen Chen, Ilsa Coleman, Hongyun Wang, Zi Fang, Shaoyong Chen et al. "Androgen receptor gene expression in prostate cancer is directly suppressed by the androgen receptor through recruitment of lysine-specific demethylase 1." Cancer cell 20, no. 4 (2011): 457-471.
9. Antonarakis, Emmanuel S., Changxue Lu, Hao Wang, Brandon Luber, Mary Nakazawa, Jeffrey C. Roeser, Yan Chen et al. "AR-V7 and resistance to enzalutamide and abiraterone in prostate cancer." New England Journal of Medicine 371, no. 11 (2014): 1028-1038.
10. Chmelar, Renée, Grant Buchanan, Eleanor F. Need, Wayne Tilley, and Norman M. Greenberg. "Androgen receptor coregulators and their involvement in the development and progression of prostate cancer." International Journal of cancer 120, no. 4 (2007): 719-733.
11. Zoubeidi, Amina, Anousheh Zardan, Eliana Beraldi, Ladan Fazli, Richard Sowery, Paul Rennie, Colleen Nelson, and Martin Gleave. "Cooperative interactions between androgen receptor (AR) and heat-shock protein 27 facilitate AR transcriptional activity." Cancer research 67, no. 21 (2007): 10455-10465.
12. Gregory, Christopher W., Bin He, Raymond T. Johnson, O. Harris Ford, James L. Mohler, Frank S. French, and Elizabeth M. Wilson. "A mechanism for androgen receptor-mediated prostate cancer recurrence after androgen deprivation therapy." Cancer research 61, no. 11 (2001): 4315-4319.
13. Qin, Jun, Hui-Ju Lee, San-Pin Wu, Shih-Chieh Lin, Rainer B. Lanz, Chad J. Creighton, Francesco J. DeMayo, Sophia Y. Tsai, and Ming-Jer Tsai. "Androgen deprivation–induced NCoA2 promotes metastatic and castration-resistant prostate cancer." The Journal of clinical investigation 124, no. 11 (2014): 5013-5026.
Radiotherapy in Prostate Cancer: Utilization in the Metastatic Setting
While external beam radiotherapy is a standard treatment option as first-line therapy for men with localized prostate cancer, it has been more recently recognized as an important component in the care of men with metastatic prostate cancer. This Center of Excellence article will explore recent evidence for the utilization of radiotherapy in the metastatic setting.
- Written by: Rashid Sayyid, MD MSc & Zachary Klaassen, MD MSc
- References:
- McAllister SS, Gifford AM, Greiner AL, et al. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell. 2008;133:994-1005.
- Boeve LMS, Hulshof MCCM, Vis AN, et al. Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the Prostate in Patients with Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial: Data from the HORRAD Trial. Eur Urol. 2019;75(3):410-418.
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet. 2018;392(10162):2353-2366.
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the prostate for men with metastatic prostate cancer in the UK and Switzerland: Long-term results from the STAMPEDE randomised controlled trial. PLoS Medicine. 2022;19(6):e1003998.
- Ali A, Hoyle A, Haran AM, et al. Association of Bone Metastatic Burden With Survival Benefit From Prostate Radiotherapy in Patients With Newly Diagnosed Metastatic Prostate Cancer: A Secondary Analysis of a Randomized Clinical Trial. JAMA Oncol. 2021;7(4):555-563.
- Fizazi K, Tran N, Fein L, et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. New Engl J Med.. 2017;377(4):352-360.
- Hoyle AP, Ali A, James ND, et al. Abiraterone in “High-” and “Low-risk” Metastatic Hormone-sensitive Prostate Cancer. Eur Urol. 2018;76(6):719-728.
- Ost P, Reynders D, Decaestecker K, et al. Surveillance or metastasis-directed therapy for oligometastatic prostate cancer recurrence: A prospective, randomized, multicenter phase II trial. J Clin Oncol. 2018;36(5):446-453.
- Phillips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020;6(5):650-659.
- Deek MP, van der Eecken K, Sutera P, et al. Long-Term Outcomes and Genetic Predictors of Response to Metastasis-Directed Therapy Versus Observation in Oligometastatic Prostate Cancer: Analysis of STOMP and ORIOLE Trials. J Clin Oncol. 2022;JCO2200644.
- Palma DA, Olson R, Harrow S, et al. Stereotactic ablative radiotherapy versus standard of care palliative treatment in patients with oligometastatic cancers (SABR-COMET): a randomised, phase 2, open-label trial. Lancet. 2019;393(10185):2051-2058.
- Palma DA, Olson R, Harrow S, et al. Stereotactic Ablative Radiotherapy for the Comprehensive Treatment of Oligometastatic Cancers: Long-Term Results of the SABR-COMET Phase II Randomized Trial. J Clin Oncol. 2020;38(25):2830-2838.
- Supiot S, Vaugier L, Pasquier D, et al. OLIGOPELVIS GETUG P07, a Multicenter Phase II Trial of Combined High-dose Salvage Radiotherapy and Hormone Therapy in Oligorecurrent Pelvic Node Relapses in Prostate Cancer. Eur Urol. 2021;80(4):405-414.
The Genetics of Prostate Cancer
Germline mutations in prostate cancer carcinogenesis
Some of the first data to delineate the value of assessment of inherited genetic changes in prostate cancer came from Pritchard and colleagues who assessed the prevalence of mutations in 20 DNA-repair genes among 692 patients with metastatic prostate cancer8. They identified such mutations in 82 men (11.8%).
- Written by: Zachary Klaassen, MD, MSc
- References:
1. Kang ZJ, Liu YF, Xu LZ, et al. The Philadelphia chromosome in leukemogenesis. Chin J Cancer 2016; 35:48.
2. An X, Tiwari AK, Sun Y, et al. BCR-ABL tyrosine kinase inhibitors in the treatment of Philadelphia chromosome positive chronic myeloid leukemia: a review. Leuk Res 2010; 34(10):1255-68.
3. Lichtenstein P, Holm NV, Verkasalo PK, et al. Environmental and heritable factors in the causation of cancer--analyses of cohorts of twins from Sweden, Denmark, and Finland. N Engl J Med 2000; 343(2):78-85.
4. Stanford JL, Ostrander EA. Familial prostate cancer. Epidemiol Rev 2001; 23(1):19-23.
5. Carter BS, Bova GS, Beaty TH, et al. Hereditary prostate cancer: epidemiologic and clinical features. J Urol 1993; 150(3):797-802.
6. Bostwick DG, Burke HB, Djakiew D, et al. Human prostate cancer risk factors. Cancer 2004; 101(10 Suppl):2371-490.
7. Alvarez-Cubero MJ, Saiz M, Martinez-Gonzalez LJ, et al. Genetic analysis of the principal genes related to prostate cancer: a review. Urol Oncol 2013; 31(8):1419-29.
8. Pritchard CC, Mateo J, Walsh MF, et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N Engl J Med 2016; 375(5):443-53.
9. Castro E, Romero-Laorden N, Del Pozo A, et al. PROREPAIR-B: A Prospective Cohort Study of the Impact of Germline DNA Repair Mutations on the Outcomes of Patients With Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol 2019; 37(6):490-503.
10. Nicolosi P, Ledet E, Yang S, et al. Prevalence of Germline Variants in Prostate Cancer and Implications for Current Genetic Testing Guidelines. JAMA Oncol 2019; 5(4):523-528.
11. Mateo J, Carreira S, Sandhu S, et al. DNA-Repair Defects and Olaparib in Metastatic Prostate Cancer. N Engl J Med 2015; 373(18):1697-708.
12. Robinson D, Van Allen EM, Wu YM, et al. Integrative clinical genomics of advanced prostate cancer. Cell 2015; 161(5):1215-1228.
13. Giri VN, Knudsen KE, Kelly WK, et al. Role of Genetic Testing for Inherited Prostate Cancer Risk: Philadelphia Prostate Cancer Consensus Conference 2017. J Clin Oncol 2018; 36(4):414-424.
14. Wallis CJ, Nam RK. Prostate Cancer Genetics: A Review. EJIFCC 2015; 26(2):79-91.
15. Ahmad AS, Vasiljevic N, Carter P, et al. A novel DNA methylation score accurately predicts death from prostate cancer in men with low to intermediate clinical risk factors. Oncotarget 2016; 7(44):71833-71840.
16. Majumdar S, Buckles E, Estrada J, et al. Aberrant DNA methylation and prostate cancer. Curr Genomics 2011; 12(7):486-505.
The Current Landscape of PSMA PET Imaging in Prostate Cancer: Advanced Prostate Cancer
- Written by: Rashid Sayyid, MD MSc, & Zachary Klaassen, MD MSc
- References:
- Deek MP, van der Eecken K, Sutera P, et al. Long-Term Outcomes and Genetic Predictors of Response to Metastasis-Directed Therapy Versus Observation in Oligometastatic Prostate Cancer: Analysis of STOMP and ORIOLE Trials. J Clin Oncol. 2022;JCO2200644.
- Philips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol. 2020;6(5):650-9.
- Kneebone A, Hruby G, Ainsworth H, et al. Stereotactic Body Radiotherapy for Oligometastatic Prostate Cancer Detected via Prostate-specific Membrane Antigen Positron Emission Tomography. Eur Urol Oncol. 2018;1(6):531-7.
- Fizazi K, Shore N, Tammela TL, et al. Nonmetastatic, Castration-Resistant Prostate Cancer and Survival with Darolutamide. N Engl J Med. 2020;383:1040–9.
- Smith MR, Saad F, Chowdhury S, et al. Apalutamide and Overall Survival in Prostate Cancer. Eur Urol. 2021;79:150–8.
- Sternberg CN, Fizazi K, Saad F, et al. Enzalutamide and Survival in Nonmetastatic, Castration-Resistant Prostate Cancer. N Engl J Med. 2020;382:2197–206.
- Fendler WP, Weber M, Iravani A, et al. Prostate-Specific Membrane Antigen Ligand Positron Emission Tomography in Men with Nonmetastatic Castration-Resistant Prostate Cancer. Clin Cancer Res. 2019;25(24):7448-54.
- Wang B, Liu C, Wei Y, et al. A Prospective Trial of 68Ga-PSMA and 18F-FDG PET/CT in Nonmetastatic Prostate Cancer Patients with an Early PSA Progression During Castration. Clin Cancer Res. 2020;26(17):4551-8.
- Fourquet A, Aveline C, Cussenot O, et al. 68 Ga-PSMA-11 PET/CT in restaging castration-resistant nonmetastatic prostate cancer: detection rate, impact on patients' disease management and adequacy of impact. Sci Rep. 2020;10(1):2104.
- Wright GL, Grob BM, Haley C, et al. Upregulation of prostate-specific membrane antigen after androgen-deprivation therapy. Urology. 1996;48:326–34.
- Evans MJ, Smith-Jones PM, Wongvipat J, et al. Noninvasive measurement of androgen receptor signaling with a positron-emitting radiopharmaceutical that targets prostate-specific membrane antigen. Proc Natl Acad Sci USA. 2011;108:9578–82.
- Aggarwal R, Wei X, Kim W, et a. Heterogeneous Flare in Prostate-specific Membrane Antigen Positron Emission Tomography Tracer Uptake with Initiation of Androgen Pathway Blockade in Metastatic Prostate Cancer. Eur Urol Oncol. 2018;1(1):78-82.
- Emmett L, Yin C, Crumbaker M, et al. Rapid Modulation of PSMA Expression by Androgen Deprivation: Serial 68Ga-PSMA-11 PET in Men with Hormone-Sensitive and Castrate-Resistant Prostate Cancer Commencing Androgen Blockade. J Nucl Med. 2019;60:950-4.
- Ettala O, Malaspina S, Tuokkola T, et al. Prospective study on the effect of shortterm androgen deprivation therapy on PSMA uptake evaluated with 68Ga-PSMA-11 PET/MRI in men with treatment-naïve prostate cancer. Eur J Nucl Med Mol Imaging. 2020;47:665–673.
- Afshar-Oromieh A, Debus N, Uhrig M, et al. Impact of long-term androgen deprivation therapy on PSMA ligand PET/CT in patients with castration-sensitive prostate cancer. Eur J Nucl Med Mol Imaging. 2018;45:2045–2054.
- Seitz AK, Rauscher I, Haller B, et al. Preliminary results on response assessment using 68Ga-HBED-CC-PSMA PET/CT in patients with metastatic prostate cancer undergoing docetaxel chemotherapy. Eur J Nucl Med Mol Imaging 2018;45:602–12.
- Grubmüller B, Razul S, Baltzer P, et al. Response assessment using [68Ga]Ga-PSMA ligand PET in patients undergoing systemic therapy for metastatic castration-resistant prostate cancer. Prostate 2020;80:74–82.
- Kallur KG, Ramachandra PG, Rajkumar K, et al. Clinical utility of gallium-68 PSMA PET/CT scan for prostate cancer. Indian J Nucl Med 2017;32:110–7.
- Fanti S, Hadaschik B, Herrmann K. Proposal for Systemic-Therapy Response-Assessment Criteria at the Time of PSMA PET/CT Imaging: The PSMA PET Progression Criteria. J Nucl Med. 2020;61(5):678-82.
Oligometastatic Prostate Cancer – Treatment of the Primary Tumor and Metastasis Directed Therapy
- Written by: Hanan Goldberg, MD
- References:
1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 2018; 68(6): 394-424.
2. Hellman S, Weichselbaum RR. Oligometastases. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 1995; 13(1): 8-10.
3. Weichselbaum RR, Hellman S. Oligometastases revisited. Nature reviews Clinical oncology 2011; 8(6): 378-82.
4. Soloway MS, Hardeman SW, Hickey D, et al. Stratification of patients with metastatic prostate cancer based on extent of disease on initial bone scan. Cancer 1988; 61(1): 195-202.
5. Weiner AB, Nettey OS, Morgans AK. Management of Metastatic Hormone-Sensitive Prostate Cancer (mHSPC): an Evolving Treatment Paradigm. Current treatment options in oncology 2019; 20(9): 69.
6. Rao A, Vapiwala N, Schaeffer EM, Ryan CJ. Oligometastatic Prostate Cancer: A Shrinking Subset or an Opportunity for Cure? American Society of Clinical Oncology educational book American Society of Clinical Oncology Annual Meeting 2019; 39: 309-20.
7. Tosoian JJ, Gorin MA, Ross AE, Pienta KJ, Tran PT, Schaeffer EM. Oligometastatic prostate cancer: definitions, clinical outcomes, and treatment considerations. Nature reviews Urology 2017; 14(1): 15-25.
8. Foster CC, Weichselbaum RR, Pitroda SP. Oligometastatic prostate cancer: Reality or figment of imagination? Cancer 2019; 125(3): 340-52.
9. Triggiani L, Alongi F, Buglione M, et al. Efficacy of stereotactic body radiotherapy in oligorecurrent and in oligoprogressive prostate cancer: new evidence from a multicentric study. British journal of cancer 2017; 116(12): 1520-5.
10. Pembroke CA, Fortin B, Kopek N. Comparison of survival and prognostic factors in patients treated with stereotactic body radiotherapy for oligometastases or oligoprogression. Radiotherapy and oncology : journal of the European Society for Therapeutic Radiology and Oncology 2018; 127(3): 493-500.
11. Jorgensen T, Muller C, Kaalhus O, Danielsen HE, Tveter KJ. Extent of disease based on initial bone scan: important prognostic predictor for patients with metastatic prostatic cancer. Experience from the Scandinavian Prostatic Cancer Group Study No. 2 (SPCG-2). European urology 1995; 28(1): 40-6.
12. Gandaglia G, Karakiewicz PI, Briganti A, et al. Impact of the Site of Metastases on Survival in Patients with Metastatic Prostate Cancer. European urology 2015; 68(2): 325-34.
13. Gakis G, Boorjian SA, Briganti A, et al. The role of radical prostatectomy and lymph node dissection in lymph node-positive prostate cancer: a systematic review of the literature. European urology 2014; 66(2): 191-9.
14. von Bodman C, Godoy G, Chade DC, et al. Predicting biochemical recurrence-free survival for patients with positive pelvic lymph nodes at radical prostatectomy. The Journal of urology 2010; 184(1): 143-8.
15. Briganti A, Karnes JR, Da Pozzo LF, et al. Two positive nodes represent a significant cut-off value for cancer specific survival in patients with node positive prostate cancer. A new proposal based on a two-institution experience on 703 consecutive N+ patients treated with radical prostatectomy, extended pelvic lymph node dissection and adjuvant therapy. European urology 2009; 55(2): 261-70.
16. Francini E, Gray KP, Xie W, et al. Time of metastatic disease presentation and volume of disease are prognostic for metastatic hormone sensitive prostate cancer (mHSPC). 2018; 78(12): 889-95.
17. Linch M, Goh G, Hiley C, et al. Intratumoural evolutionary landscape of high-risk prostate cancer: the PROGENY study of genomic and immune parameters. Annals of oncology : official journal of the European Society for Medical Oncology 2017; 28(10): 2472-80.
18. Gundem G, Van Loo P, Kremeyer B, et al. The evolutionary history of lethal metastatic prostate cancer. Nature 2015; 520(7547): 353-7.
19. Cooper CS, Eeles R, Wedge DC, et al. Analysis of the genetic phylogeny of multifocal prostate cancer identifies multiple independent clonal expansions in neoplastic and morphologically normal prostate tissue. Nature genetics 2015; 47(4): 367-72.
20. Larbi A, Dallaudiere B, Pasoglou V, et al. Whole body MRI (WB-MRI) assessment of metastatic spread in prostate cancer: Therapeutic perspectives on targeted management of oligometastatic disease. The Prostate 2016; 76(11): 1024-33.
21. Graziani T, Ceci F, Castellucci P, et al. (11)C-Choline PET/CT for restaging prostate cancer. Results from 4,426 scans in a single-centre patient series. European journal of nuclear medicine and molecular imaging 2016; 43(11): 1971-9.
22. McAllister SS, Gifford AM, Greiner AL, et al. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell 2008; 133(6): 994-1005.
23. Kaplan RN, Riba RD, Zacharoulis S, et al. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 2005; 438(7069): 820-7.
24. Bayne CE, Williams SB, Cooperberg MR, et al. Treatment of the Primary Tumor in Metastatic Prostate Cancer: Current Concepts and Future Perspectives. European urology 2016; 69(5): 775-87.
25. Locke JA, Dal Pra A, Supiot S, Warde P, Bristow RG. Synergistic action of image-guided radiotherapy and androgen deprivation therapy. Nature reviews Urology 2015; 12(4): 193-204.
26. Kalina JL, Neilson DS, Comber AP, et al. Immune Modulation by Androgen Deprivation and Radiation Therapy: Implications for Prostate Cancer Immunotherapy. Cancers (Basel) 2017; 9(2): 13.
27. Heidenreich A, Pfister D, Porres D. Cytoreductive radical prostatectomy in patients with prostate cancer and low volume skeletal metastases: results of a feasibility and case-control study. The Journal of urology 2015; 193(3): 832-8.
28. Bianchini D, Lorente D, Rescigno P, et al. Effect on Overall Survival of Locoregional Treatment in a Cohort of De Novo Metastatic Prostate Cancer Patients: A Single Institution Retrospective Analysis From the Royal Marsden Hospital. Clinical genitourinary cancer 2017; 15(5): e801-e7.
29. Gratzke C, Engel J, Stief CG. Role of radical prostatectomy in metastatic prostate cancer: data from the Munich Cancer Registry. European urology 2014; 66(3): 602-3.
30. Culp SH, Schellhammer PF, Williams MB. Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study. European urology 2014; 65(6): 1058-66.
31. O'Shaughnessy MJ, McBride SM, Vargas HA, et al. A Pilot Study of a Multimodal Treatment Paradigm to Accelerate Drug Evaluations in Early-stage Metastatic Prostate Cancer. Urology 2017; 102: 164-72.
32. Satkunasivam R, Kim AE, Desai M, et al. Radical Prostatectomy or External Beam Radiation Therapy vs No Local Therapy for Survival Benefit in Metastatic Prostate Cancer: A SEER-Medicare Analysis. The Journal of urology 2015; 194(2): 378-85.
33. Loppenberg B, Dalela D, Karabon P, et al. The Impact of Local Treatment on Overall Survival in Patients with Metastatic Prostate Cancer on Diagnosis: A National Cancer Data Base Analysis. European urology 2017; 72(1): 14-9.
34. Boeve LMS, Hulshof M, Vis AN, et al. Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the Prostate in Patients with Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial: Data from the HORRAD Trial. European urology 2019; 75(3): 410-8.
35. Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet (London, England) 2018; 392(10162): 2353-66.
36. Sweeney CJ, Chen Y-H, Carducci M, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer. New England Journal of Medicine 2015; 373(8): 737-46.
37. Burdett S, Boeve LM, Ingleby FC, et al. Prostate Radiotherapy for Metastatic Hormone-sensitive Prostate Cancer: A STOPCAP Systematic Review and Meta-analysis. European urology 2019; 76(1): 115-24.
38. Potters L, Kavanagh B, Galvin JM, et al. American Society for Therapeutic Radiology and Oncology (ASTRO) and American College of Radiology (ACR) practice guideline for the performance of stereotactic body radiation therapy. International journal of radiation oncology, biology, physics 2010; 76(2): 326-32.
39. De Bleser E, Tran PT, Ost P. Radiotherapy as metastasis-directed therapy for oligometastatic prostate cancer. Current opinion in urology 2017; 27(6): 587-95.
40. Decaestecker K, De Meerleer G, Lambert B, et al. Repeated stereotactic body radiotherapy for oligometastatic prostate cancer recurrence. Radiat Oncol 2014; 9: 135-.
41. Palma DA, Salama JK, Lo SS, et al. The oligometastatic state - separating truth from wishful thinking. Nature reviews Clinical oncology 2014; 11(9): 549-57.
42. Riva G, Marvaso G, Augugliaro M, et al. Cytoreductive prostate radiotherapy in oligometastatic prostate cancer: a single centre analysis of toxicity and clinical outcome. Ecancermedicalscience 2017; 11: 786.
43. Ost P, Reynders D, Decaestecker K, et al. Surveillance or Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2018; 36(5): 446-53.
44. Nguyen PL, Alibhai SM, Basaria S, et al. Adverse effects of androgen deprivation therapy and strategies to mitigate them. European urology 2015; 67(5): 825-36.
45. Duchesne GM, Woo HH, Bassett JK, et al. Timing of androgen-deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01-03 [TOAD]): a randomised, multicentre, non-blinded, phase 3 trial. The Lancet Oncology 2016; 17(6): 727-37.
46. Ost P, Bossi A, Decaestecker K, et al. Metastasis-directed therapy of regional and distant recurrences after curative treatment of prostate cancer: a systematic review of the literature. European urology 2015; 67(5): 852-63.
47. Steuber T, Jilg C, Tennstedt P, et al. Standard of Care Versus Metastases-directed Therapy for PET-detected Nodal Oligorecurrent Prostate Cancer Following Multimodality Treatment: A Multi-institutional Case-control Study. European urology focus 2018.
48. Siva S, Bressel M, Murphy DG, et al. Stereotactic Abative Body Radiotherapy (SABR) for Oligometastatic Prostate Cancer: A Prospective Clinical Trial. European urology 2018; 74(4): 455-62.
49. Ploussard G, Gandaglia G, Borgmann H, et al. Salvage Lymph Node Dissection for Nodal Recurrent Prostate Cancer: A Systematic Review. European urology 2018.
50. Zattoni F, Nehra A, Murphy CR, et al. Mid-term Outcomes Following Salvage Lymph Node Dissection for Prostate Cancer Nodal Recurrence Status Post-radical Prostatectomy. European urology focus 2016; 2(5): 522-31.
51. Fossati N, Suardi N, Gandaglia G, et al. Identifying the Optimal Candidate for Salvage Lymph Node Dissection for Nodal Recurrence of Prostate Cancer: Results from a Large, Multi-institutional Analysis. European urology 2019; 75(1): 176-83.
52. Standard Systemic Therapy With or Without Definitive Treatment in Treating Participants With Metastatic Prostate Cancer. 2019. https://clinicaltrials.gov/ct2/show/NCT03678025. (accessed August 8th 2019).
53. Stereotactic Body Radiation for Prostate Oligometastases (ORIOLE). NCT02680587. 2019. https://clinicaltrials.gov/ct2/show/ (accessed August 8th 2019).
54. Radwan N, Phillips R, Ross A, et al. A phase II randomized trial of Observation versus stereotactic ablative RadiatIon for OLigometastatic prostate CancEr (ORIOLE). BMC cancer 2017; 17(1): 453.
55. Rowe SP, Macura KJ, Mena E, et al. PSMA-Based [(18)F]DCFPyL PET/CT Is Superior to Conventional Imaging for Lesion Detection in Patients with Metastatic Prostate Cancer. Mol Imaging Biol 2016; 18(3): 411-9.
56. Sooriakumaran P. Testing radical prostatectomy in men with prostate cancer and oligometastases to the bone: a randomized controlled feasibility trial. BJU international 2017; 120(5b): E8-e20.
The Current Landscape of Metastatic Castration-Resistant Prostate Cancer: Radioligand Therapy
- Written by: Rashid Sayyid, MD MSc, & Zachary Klaassen, MD MSc
- References:
- Sartor O. Isotope Therapy for Castrate-Resistant Prostate Cancer: Unique Sequencing and Combinations. Cancer J 2016; 22(5):342-346.
- Ye X, Sun D, Lou C. Comparison of the efficacy of strontium-89 chloride in treating bone metastasis of lung, breast, and prostate cancers. J Cancer Res Ther 2018; 14(Supplement):S36-S40.
- James N, Pirrie S, Pope A, et al. TRAPEZE: a randomised controlled trial of the clinical effectiveness and cost-effectiveness of chemotherapy with zoledronic acid, strontium-89, or both, in men with bony metastatic castration-refractory prostate cancer. Health Technol Assess 2016; 20(53):1-288.
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213-223.
- Chang SS. Overview of Prostate-Specific Membrane Antigen. Rev Urol. 2004;6(Suppl 10):S13-S18.
- Hofman MS, Violet J, Hicks RJ, et al. [177Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19:825-833.
- Seifert R, Kessel K, Schlack K, Weckesser M, Bogemann M, Rahbar K. Radioligand therapy using [(177)Lu]Lu-PSMA-617 in mCRPC: a pre-VISION single-center analysis. Eur J Nucl Med Mol Imaging. 2020;47(9):2106-2112.
- Hofman MS, Emmett L, Sandhu S, et al. [177Lu]Lu-PSMA-617 versus cabazitaxel in patients with metastatic castration-resistant prostate cancer (TheraP): a randomised, open-label, phase 2 trial. Lancet. 2021;397(10276):797-804.
- Gaertner FC, Halabi K, Ahmadzadehfar H, et al. Uptake of PSMA-ligands in normal tissues is dependent on tumor load in patients with prostate cancer. Oncotarget. 2017;8(33):55094-55103.
- Sartor O, de Bono J, Chi KN, et al. Lutetium-177–PSMA-617 for Metastatic Castration-Resistant Prostate Cancer. N Engl J Med. 2021;385:1901-1103.
- Hartrampf PE, Seitz AK, Weinzierl F, et al. Baseline clinical characteristics predict overall survival in patients undergoing radioligand therapy with [ 177 Lu]Lu-PSMA I&T during long-term follow-up. Eur J Ncul Med Mol Imaging. 2022.
Prostate Cancer Survivorship
Physical Side Effects
Urinary Dysfunction
Urinary dysfunction is a side effect of both surgical and radiotherapy (RT) for local treatment of prostate cancer (PCa). Surgical side effects typically include a period of urinary incontinence for several months postoperatively followed by a degree of stress urinary incontinence that may persist for months or even years. RT-induced urinary dysfunction typically manifests as bladder irritability/overactivity either during treatment or shortly thereafter. Longer-term urinary dysfunction issues after RT may include urethral strictures necessitating periodic interventions and/or catheterization.
The ProtecT trial randomized 1,643 men from 1999 to 2009 to undergoing either active monitoring (n=545), surgery (n=553), or RT (n=545), finding that at a median 10 years of follow-up, PCa-specific mortality was low irrespective of treatment.2 As part of this trial, patient-reported outcomes were collected and have now become one of the benchmarks for counseling patients with regards to long-term side effects of treatment for localized PCa treatment.3 Questionnaires were completed at the time of diagnosis, at 6 and 12 months after randomization, and annually thereafter. Patients completed validated measures that assessed urinary, bowel, and sexual function and specific effects on quality of life, anxiety, and depression, and general health. The rate of questionnaire completion during follow-up was outstanding at >85% for most measures. Regarding urinary dysfunction, radical prostatectomy (RP) had the greatest negative effect on urinary continence, and although there was some recovery over time, these patients remained worse throughout follow-up compared to patients undergoing active monitoring or RT. Interestingly, RT had little effect on urinary incontinence, and there was a gradual decrease in urinary function over time for the men undergoing active monitoring. Urinary voiding and nocturia were worse in the radiotherapy group at 6 months but then mostly recovered and were similar to the other groups after 12 months. Urinary incontinence has been cited as being the most important factor for decision regret among receiving local therapy for PCa and may be incompletely explained/discussed with ~80% of patients prior to undergoing treatment.4
Sexual Dysfunction
Similar to urinary dysfunction, sexual dysfunction is a common side effect of localized therapy for PCa. Patients undergoing RP will suffer a degree of sexual dysfunction in the immediate postoperative period with a degree of recovering over 12-24 months after surgery. Many studies have been published assessing predictors of postoperative recovery of sexual function, commonly highlighting younger age and adequate function pre-operatively as predictors of post-operative recovery. Men undergoing RT, similar to urinary dysfunction, will not notice an immediate effect on sexual function during the treatment phase, but generally, suffer sexual dysfunction in the years post-radiation.
In the ProtecT trial, RP incurred the greatest degree of sexual dysfunction among all three treatment arms, with some recovery of function over time.3 The negative effect of RT on sexual function was greatest at 6 months, but sexual function then recovered somewhat and was stable thereafter. Sexual dysfunction also declined in the active monitoring group over time.
Primarily secondary to the sexual side effects of localized treatment for PCa, many cancer centers now have fellowship-trained experts that see these patients concomitantly with the oncologist. There are a variety of treatment options offered, including oral PDE-5 inhibitors (sildenafil, tadalafil, etc.), intracavernosal injection therapy, and penile prosthetics.
Bowel Dysfunction
Bowel dysfunction is typically low for patients undergoing RP or active surveillance (AS) but may be a detrimental side effect among men undergoing RT. In the ProtecT trial, bowel function was worse in the RT group at 6 months than in the other groups but then recovered somewhat, except for the increasing frequency of bloody stools; bowel function was unchanged in the active monitoring and RP groups.3
Bowel dysfunction and rectal toxicity has improved with the recent FDA approval of hydrogel rectal spacers. Prior to RT, patients may have a hydrogel rectal spacer (SpaceOAR®) placed in a transperineal fashion in the fat between the rectum and Denonvilliers' fascia. In the pivotal clinical trial assessing hydrogel spacers, 114 patients were enrolled between 2010 and 2011 with 54 patients selected for a hydrogel injection before the beginning of RT.5 Patients were surveyed at various time-points with the EPIC PCa questionnaire – among patients treated with a hydrogel spacer, mean bowel function and bother score changes of >5 points in comparison with baseline levels were found only at the end of RT (10-15 points; p < 0.01). Mean bowel bother score changes of 21 points at the end of RT, 8 points at 2 months, 7 points at 17 months, and 6 points at 63 months after RT were found for patients treated without a spacer. These bowel quality of life results have given hydrogel spacers an option among patients considering RT.
Other health-related effects
There is evidence that both RT and androgen deprivation therapy (ADT) may contribute to the development of coronary heart disease, sudden cardiac death, myocardial infarction, and skeletal-related events such as fracture.6
Psychological Side Effects
Depression and AnxietyDepression is the most common psychiatric comorbidity among cancer patients, including patients with PCa. Ravi et al.7 previously utilized the SEER-Medicare database to assess the burden of mental health issues (anxiety, major depressive disorder, suicide) in patients with localized PCa. Among 50,586 men >65 years of age without a diagnosis of mental illness, 20.4% of men developed a mental illness with a median 55-month follow-up. Interestingly, patients undergoing WW (29.7%) and RT (29.0%) had a significantly increased incidence of mental illness compared to patients undergoing RP (22.6%; p<0.001). A systematic review of depression and anxiety in patients with PCa identified 27 articles comprising 4,494 patients.8 The meta-analysis of prevalence rates identified pretreatment prevalence of depression of 17.27% (95% confidence interval (CI) 15.06%-19.72%), on-treatment prevalence of 14.70% (95% CI 15.06%-19.72%) and post-treatment prevalence of 18.44% (95% CI 15.18%-22.22%). For anxiety, pretreatment prevalence was 27.04% (95% CI 24.26%-30.01%), on-treatment was 15.09% (95% CI 12.15%-18.60%) and post-treatment was 18.49% (95% CI 13.81%-24.31%). For patients undergoing AS, nearly one-third of patients (29%) report cancer-specific anxiety in the year following diagnosis.9 Interestingly, over time, this anxiety decreased significantly.
There is also increasing evidence that ADT for locally advanced and metastatic PCa is associated with depression. A study from 2016 using SEER-Medicare data found that men that received ADT, compared with patients who did not receive ADT, had higher 3-year cumulative incidences of depression (7.1% v 5.2), inpatient psychiatric treatment (2.8% v 1.9%), and outpatient psychiatric treatment (3.4% v 2.5%).10 Furthermore, the risk of depression increased with the duration of ADT, from 12% with ≤ 6 months of treatment, 26% with 7 to 11 months of treatment, to 37% with ≥ 12 months of treatment. A recent meta-analysis of 18 studies among 168,756 men found that ADT use conferred a 41% increased risk of depression (RR 1.41, 95%CI 1.18-1.70).11 These results were consistent when limiting the analysis to studies in localized disease (relative risk (RR) 1.85, 95%CI 1.20-2.85). Interestingly, this analysis did not find an association for continuous ADT with depression risk compared to intermittent ADT (RR 1.00, 95%CI 0.50-1.99).
Suicidal Risk
Patients with PCa have been shown to be at increased risk of suicide across several population-level studies. In a SEER analysis assessing suicide risk among patients with genitourinary malignancies from 1988-2010, Klaassen et al.12 found an age-adjusted standardized mortality ratio (SMR) of 1.37 for patients with PCa (95%CI, 0.99-1.86) Increasing age, metastatic disease and Caucasian race were risk factors for suicide among these patients. Interestingly, even patients >15 years after diagnosis were at increased risk of suicide compared to the general population (SMR 1.84, 95%CI 1.39-2.41). In an assessment of PCa suicidal risk compared to individuals with other malignancies, Dalela et al.13 found that risk of suicidal death was no different in men with PCa (1,165 [0.2%]) compared to men with other cancers (2,232 [0.2%]), However, within the first year of diagnosis, men with PCa had an increased risk of suicide (absolute risk reduction (ARR) 3.98, 95% CI 3.02-5.23 0-3 months after diagnosis). Furthermore, men with non-metastatic PCa who were Caucasian, uninsured, or recommended but did not receive treatment (hazard ratio (HR) vs treated 1.44, 95%CI 1.20-1.72) were at increased risk of suicidal death.
A meta-analysis of observational studies assessing incidence and risk factors of suicide after PCa diagnosis was recently published.14 This study included 8 observational studies involving 1,281,393 men diagnosed with PCa and 842,294 matched PCa-free men. Guo et al. found an overall increased relative risk of suicide of 2.01 (95% CI 1.52-2.64) among men diagnosed with PCa compared with those without PCa during the first year after diagnosis, particularly during the first 6 months after diagnosis (RR 2.24, 95%CI 1.77-2.85). Additionally, PCa patients were at an increased risk of suicide among men aged 75 years or older (RR 1.51, 95% CI 1.04-2.18) and for those treated with ADT (RR 1.80, 95% CI 1.54-2.12).
Until recently, all population-level studies assessing risk of suicide among PCa patients have not accounted for psychiatric comorbidities at the time of diagnosis. This is important, considering that being unable to adjust for psychiatric comorbidities makes it impossible to assess the true risk associated with a PCa diagnosis on suicidal risk. At the AUA 2019 annual meeting, Klaassen et al.15 presented data assessing all residents of Ontario, Canada diagnosed with either prostate, bladder or kidney cancer (1997-2014). Each patient was assigned a psychiatric utilization gradient (PUG) score in the five years prior to cancer diagnosis: 0 (none), 1 (outpatient), 2 (emergency department), 3 (hospital admission). Non-cancer controls were matched 4:1 to cancer patients based on sociodemographic variables and a marginal cause-specific hazard model was used to assess the effect of cancer on the risk of suicidal death. Among 191,068 patients included (137,699 PCa, 29,884 bladder cancer, 23,485 kidney cancer), 109,154 (57.1%) were PUG score 0, 79,553 (41.6%) PUG score 1, 1,596 (0.84%) PUG score 2, and 765 (0.40%) PUG score 3. Patients with genitourinary cancer had a higher risk of dying of suicide compared to controls (HR 1.16, 95%CI 1.00-1.36). Specifically, among individuals with PUG score 0, those with cancer were significantly more likely to die of suicide compared to patients without cancer (HR 1.39, 95%CI 1.12-1.74).
Guideline Recommendations
The Commission on Cancer requires cancer programs to develop and implement processes to monitor formation and dissemination of a survivorship care plan for all cancer patients with stage I-III disease treated with curative intent, and to have this plan in place within 1-year of diagnosis of cancer and no later than 6 months after completing adjuvant therapy.16 Guideline recommendations for PCa survivorship have primarily been driven by the American Cancer Society (ACS) and the American Society of Clinical Oncology (ASCO). The ACS noted in their 2014 guideline that survivorship should promote comprehensive follow-up care and optimal health and quality of life for the post-treatment PCa survivor.17 The guidelines also address health promotion, surveillance for PCa recurrence, screening for second primary cancers, long-term and late effects assessment and management, psychosocial issues, and care coordination among the oncology team, primary care clinicians, and non-oncology specialists. Subsequently, the ASCO Endorsement Panel reviewed the ACS guidelines, endorsing these guidelines with the following recommendations:18• Measure PSA level every 6 to 12 months for the first 5 years and then annually, considering more frequent evaluation in men at high risk for recurrence and in candidates for salvage therapy.
• Refer survivors with elevated or increasing PSA levels back to their primary treating physician for evaluation and management.
• Adhere to ACS guidelines for the early detection of cancer.
• Assess and manage physical and psychosocial effects of PCa and its treatment.
• Annually assess for the presence of long-term or late effects of PCa and its treatment.
Screening Measures
There are several screening tools to assess for quality of life, depression and suicidal risk. A study from 2017 assessed differences in the scores, relative severity and major depressive disorder from three standardized self-report scales for depression in PCa patients [The Hospital Anxiety and Depression Scale Depression subscale (HADS-D), the Self-rating Depression Scale (SDS) and the Patient Health Questionnaire (PHQ-9) for depression].19 Among 138 PCa patients, despite significant correlations between the total scores from the three scales, severity classification differed across the three scales. Furthermore, there was considerable underestimation of depression by the HADS-D compared to the PHQ-9 and a similar tendency for the SDS. This study highlights that scale construction and depression items included can produce different results across scales, making inter-study comparisons difficult. Despite these findings, we recommend that at minimum oncologists should be using at least one depression index to assess patient well-being at each clinic visit.In addition to the aforementioned HADS-D, SDS, and PHQ-9 metrics, the National Comprehensive Cancer Network (NCCN) provides a guideline for identifying and explaining risk factors in patients with cancer, in addition to providing a “distress thermometer”. The NCCN defines distress, in the setting of cancer, as a multifactorial emotional experience of a psychological, social, and/or spiritual nature that may interfere with the ability to cope effectively with the diagnosis.20 Distress can range from sadness and fear to more disabling symptoms such as anxiety and depression. Furthermore, the time periods at which patients are at increased vulnerability begin with the realization of a suspicious symptom, all the way through to failure/disease recurrence and near the end of life. The NCCN recommends screening all patients for distress to recognize, monitor, and treat patients effectively.20
Previous work has also suggested that screening for depression and erectile dysfunction may be a way to decrease suicidal risk among PCa patients.21 A proposed algorithm allows for an initial evaluation with the EPIC-CP and PHQ-9 tools to assess for health-related quality of life and depression, respectively. If the EPIC-CP or PHQ-9 are negative for depression or erectile dysfunction, these tools should still be used at each visit to regularly evaluate patients. If EPIC-CP or PHQ-9 suggest problems with depression or erectile dysfunction, then an 8-question suicidal ideation questionnaire (adapted from Recklitis et al.22) should be completed. If the suicidal ideation questionnaire demonstrates any level of suicidal ideation, clinicians should make an urgent referral for psychiatric evaluation. This is particularly true when the patient has the concomitant high-risk suicidal risk profile of being elderly, white, single, or with high-risk or disease progression. Given that, at maximum, the patient must answer a 27-point composite questionnaire, this should be feasible in the busy clinical setting and can be provided to the patient at appointment check-in and completed in the waiting room before the physician-patient encounter. Regardless of the results from these screening tools, if any member of the healthcare team has an index of suspicion for suicidal ideation, the physician should immediately make a referral for psychiatric evaluation.
Conclusions
With nearly 3 million men in the United States living with PCa, survivorship programs are now mandated by the Commission on Cancer and play an integral role in health and well-being of men with PCa. In addition to the physical side effects of treatment that should be addressed at each clinic visit, there are crucial psychiatric side effects, including depression, anxiety, and suicidal ideation that should be screened for and recognized by all members of the healthcare team.Published Date: December 2019
- Written by: Zachary Klaassen, MD, MSc
- References:
1. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019;69(1):7-34.
2. Hamdy FC, Donovan JL, Lane JA, et al. 10-Year Outcomes after Monitoring, Surgery, or Radiotherapy for Localized Prostate Cancer. N Engl J Med. 2016;375(15):1415-1424.
3. Donovan JL, Hamdy FC, Lane JA, et al. Patient-Reported Outcomes after Monitoring, Surgery, or Radiotherapy for Prostate Cancer. N Engl J Med. 2016;375(15):1425-1437.
4. Albkri A, Girier D, Mestre A, Costa P, Droupy S, Chevrot A. Urinary Incontinence, Patient Satisfaction, and Decisional Regret after Prostate Cancer Treatment: A French National Study. Urol Int. 2018;100(1):50-56.
5. Pinkawa M, Berneking V, Schlenter M, Krenkel B, Eble MJ. Quality of Life After Radiation Therapy for Prostate Cancer With a Hydrogel Spacer: 5-Year Results. Int J Radiat Oncol Biol Phys. 2017;99(2):374-377.
6. Wallis CJ, Mahar AL, Satkunasivam R, et al. Cardiovascular and Skeletal-related Events Following Localized Prostate Cancer Treatment: Role of Surgery, Radiotherapy, and Androgen Deprivation. Urology. 2016;97:145-152.
7. Ravi P, Karakiewicz PI, Roghmann F, et al. Mental health outcomes in elderly men with prostate cancer. Urol Oncol. 2014;32(8):1333-1340.
8. Watts S, Leydon G, Birch B, et al. Depression and anxiety in prostate cancer: a systematic review and meta-analysis of prevalence rates. BMJ Open. 2014;4(3):e003901.
9. Marzouk K, Assel M, Ehdaie B, Vickers A. Long-Term Cancer Specific Anxiety in Men Undergoing Active Surveillance of Prostate Cancer: Findings from a Large Prospective Cohort. J Urol. 2018;200(6):1250-1255.
10. Dinh KT, Reznor G, Muralidhar V, et al. Association of Androgen Deprivation Therapy With Depression in Localized Prostate Cancer. J Clin Oncol. 2016;34(16):1905-1912.
11. Nead KT, Sinha S, Yang DD, Nguyen PL. Association of androgen deprivation therapy and depression in the treatment of prostate cancer: A systematic review and meta-analysis. Urol Oncol. 2017;35(11):664 e661-664 e669.
12. Klaassen Z, Jen RP, DiBianco JM, et al. Factors associated with suicide in patients with genitourinary malignancies. Cancer. 2015;121(11):1864-1872.
13. Dalela D, Krishna N, Okwara J, et al. Suicide and accidental deaths among patients with non-metastatic prostate cancer. BJU Int. 2016;118(2):286-297.
14. Guo Z, Gan S, Li Y, et al. Incidence and risk factors of suicide after a prostate cancer diagnosis: a meta-analysis of observational studies. Prostate Cancer Prostatic Dis. 2018;21(4):499-508.
15. Klaassen Z, Wallis CJ, Goldberg H, et al. Utilization of Psychiatric Resources Prior to Genitourinary (GU) Cancer Diagnosis: Implications for Survival Outcomes. AUA 2019. 2019.
16. Fashoyin-Aje LA, Martinez KA, Dy SM. New patient-centered care standards from the commission on cancer: opportunities and challenges. J Support Oncol. 2012;10(3):107-111.
17. Skolarus TA, Wolf AM, Erb NL, et al. American Cancer Society prostate cancer survivorship care guidelines. CA Cancer J Clin. 2014;64(4):225-249.
18. Resnick MJ, Lacchetti C, Bergman J, et al. Prostate cancer survivorship care guideline: American Society of Clinical Oncology Clinical Practice Guideline endorsement. J Clin Oncol. 2015;33(9):1078-1085.
19. Sharpley CF, Bitsika V, Christie DR, Hunter MS. Measuring depression in prostate cancer patients: does the scale used make a difference? Eur J Cancer Care (Engl). 2017;26(1).
20. National Comprehensive Cancer N. Distress management. Clinical practice guidelines. J Natl Compr Canc Netw. 2003;1(3):344-374.
21. Klaassen Z, Arora K, Wilson SN, et al. Decreasing suicide risk among patients with prostate cancer: Implications for depression, erectile dysfunction, and suicidal ideation screening. Urol Oncol. 2018;36(2):60-66.
22. Recklitis CJ, Zhou ES, Zwemer EK, Hu JC, Kantoff PW. Suicidal ideation in prostate cancer survivors: understanding the role of physical and psychological health outcomes. Cancer. 2014;120(21):3393-3400.


