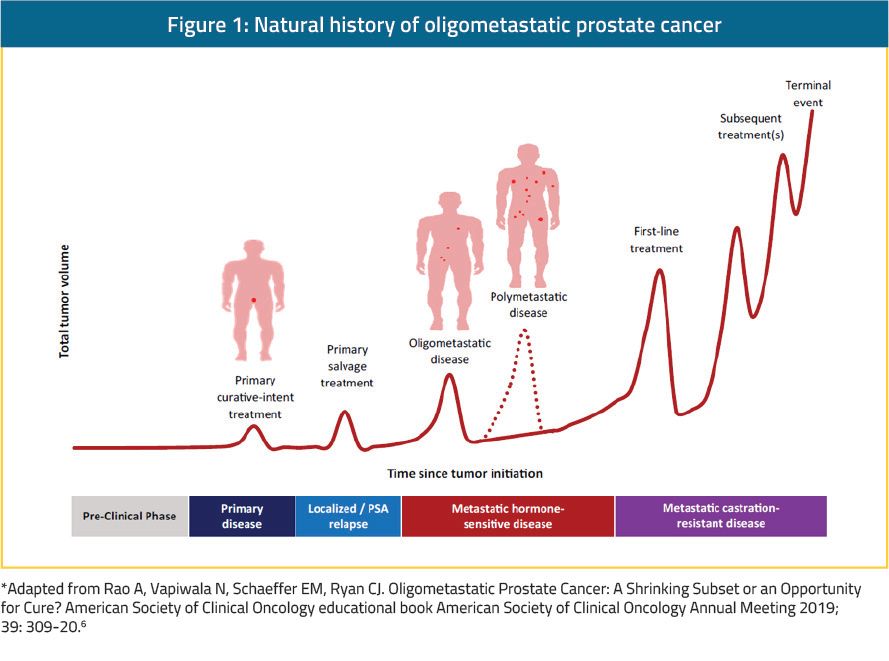
There are three distinct entities of oligometastatic PCa potentially having a different biologic signature.7 These include the:
- Oligorecurrent PCa occurring after primary localized therapy to the prostate (either radical prostatectomy [RP] or radiotherapy [RT])8
- De novo oligometastatic PCa that has spread before any definitive therapy to the primary tumor.8 In this entity, the primary tumor would need to be managed in addition to distant lesions
- Oligoprogression PCa – defined as widespread metastases with only a few sites of progression on systemic therapy9, 10, probably harboring the worst prognosis out of the three distinct entities9
Genomics of oligometastatic prostate cancer
Polyclonal PCa tumors were found to be associated with a higher risk of metastatic disease.8 In addition, the phenomenon of nonlinear clonal evolution in metastatic sites, defined as clones from metastatic sites being able to seed other sites, including the primary tumor, has been shown in PCa.17-19 In a study assessing samples of primary and metastatic tumors of patients who were treated with stereotactic RT, different microRNA expression profiles were shown between patient samples who developed oligometastatic vs. polymetastatic disease. The authors also found that miRNA-200c enhancement in the oligometastatic cell line was associated with polymetastatic disease progression. This suggests that oligometastatic disease may have a potentially less diverse and differing landscape than polymetastatic disease and the primary tumor.6Oligometastatic disease in imaging modalities
Importantly, the current standard practice includes the use of conventional imaging modalities to define the extent of metastatic disease. These include using computed tomography (CT) (sensitivity of 70-80%) and technetium bone scans (sensitivity of 60-80%). These imaging tests are widely available, with a modest cost, and have been incorporated into clinical practice and guidelines for decades. However, newly developed imaging modalities, especially positron emission tomography (PET) with various radiotracers may potentially enhance our ability to identify an occult metastatic disease and could lead to an increased incidence of oligometastatic and polymetastatic disease. Common radiotracers used for PCa include choline, 1-amino-3-fluorocyclobutane-1-carboxylic acid (fluciclovine), prostate-specific membrane antigen (PSMA) (Figure 2), 18-fluorodeoxyglucose, and NaF.6 Whole-body magnetic resonance imaging (MRI) has also shown initial promise as a modality for assessing systemic disease.20 MRI may play a role in appropriate patient selection for aggressive multimodality therapy.6 However, MRI does have some noteworthy limitations, including resource- and time-utilizing protocols, variability in scanner performances, significant artifacts, a substantial-high cost, and inter-reader variation.6 The advances and novel imaging being introduced will ultimately result in patients who were initially thought to be non-metastatic, to be defined as oligometastatic patients, and patients who were considered oligometastatic to be redefined as polymetastatic.21 It is clear that the prevalence of the oligometastatic disease will continue to change with advances in imaging and treatments for localized disease.6
Local treatment of the prostate in oligometastatic disease
There have been some data suggesting that the primary tumor in PCa plays a substantial role in disease progression even after and despite the development of metastatic disease.22-24 This has initiated investigational efforts on the potential benefit of local treatment in oligometastatic PCa patients. The most likely mechanism behind the outcome benefit for the treatment of the primary tumor in oligometastatic patients is the reduction of tumor burden. Furthermore, for RT combined with ADT, there is a synergistic effect resulting in double-strand DNA breaks in cancer cells with resultant apoptosis,25 and an immunomodulatory effect of this combination.26 Additionally, the systemic therapies given for metastatic PCa are noncurative and are associated with significant toxicities. Using therapies aimed at the primary tumor may enable a subset of patients to delay or interrupt systemic therapy, decrease some of the adverse effects and improve the patient’s quality of life.6Radical prostatectomy in oligometastatic disease
There are several retrospective studies, including population-level studies showing that surgical removal of the prostate in oligometastatic patients caused an improvement in several distinct outcomes, including delay in castration resistance PCa (CRPC) development in patients with bony metastatic disease,27 progression-free survival (PFS), CSS, and OS.12, 27-30 To date, there are no published prospective studies ascertaining the benefit of this specific treatment, but there are several ongoing trials which will be mentioned later. There are also retrospective data supporting the role of intraoperative regional lymph-node dissection in these patients.31 Although these retrospective data have been used for hypothesis generation, they are most likely prone to selection bias as included men had to be fit enough to undergo surgery.5Radiotherapy in oligometastatic disease
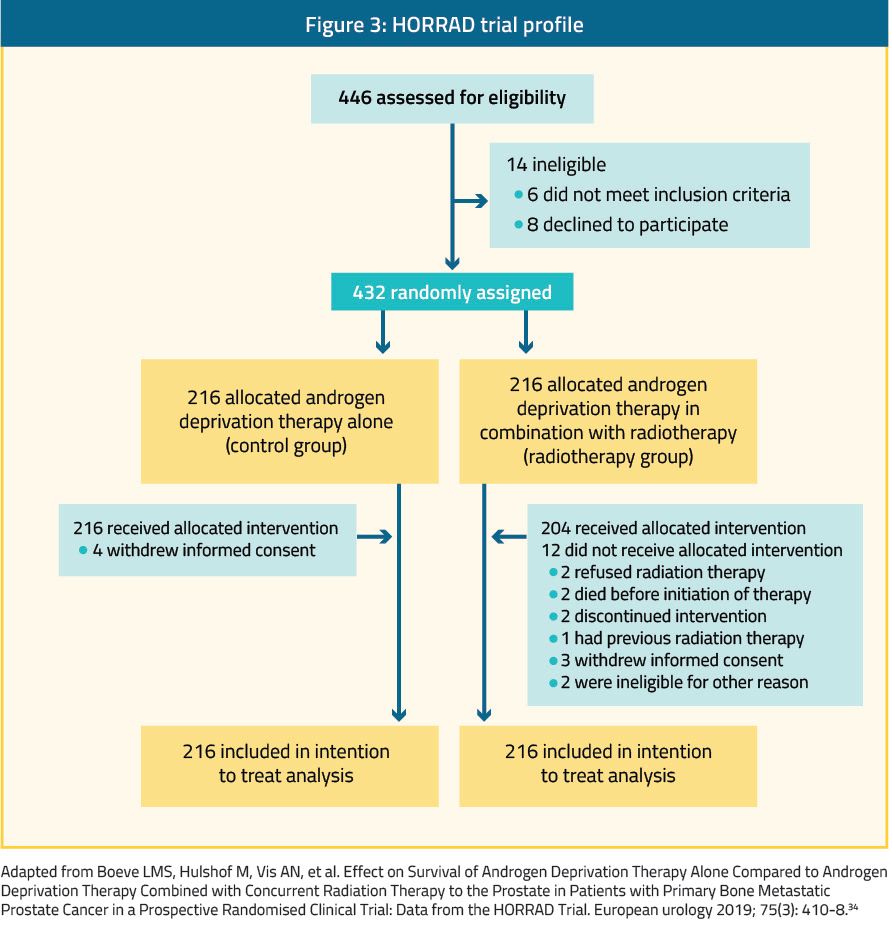
Retrospective analyses of large cancer datasets suggested a benefit to radiotherapy for the primary tumor in mHSPC.32, 33 Additionally, there have been two prospective randomized trials published in the last year, evaluating the added benefit of RT to the primary tumor with the standard of care treatment in patients with de novo mHSPC.The first was the HORRAD trial, a multicenter, randomized controlled trial of 432 patients with PSA higher than 20 ng/mL and bone-predominant metastatic disease (Figure 3).34 Patients were randomly assigned 1:1 to external beam RT (70 Gy in 35 fractions or 57.76 Gy in 19 fractions) to the prostate with ADT (RT group) or ADT alone (control group). Pelvic lymph-nodes were not included in the radiation field. This was a negative trial as no benefit was shown with the addition of RT. Median OS was 45 months (95% CI, 40.4 to 49.6 months) in the RT group compared to 43 months (95% CI, 32.6–53.4 months) in the control group (adjusted HR, 1.11; 95% CI, 0.87–1.43; p=0.4). Survival outcomes were comparable between the two groups for patients with oligometastatic disease (fewer than five lesions) with an HR 0.68, 95% CI, 0.42–1.10), and also for those with five to 15 lesions (HR, 1.18; 95% CI,0.74–1.89), and more than 15 lesions (HR, 0.93; 95% CI,0.66–1.32).34 Important limitations of this trial included the lack of assessment of visceral combined with bone metastatic disease, as only bone-metastatic patients were included. Additionally, the median PSA was very high (142 ng/ml), suggesting that patients had much higher metastatic burden than originally assumed. Lastly, the RT regimen used was lower (70 Gy) than the current standard practice of (72-78 Gy), and lymph nodes were not treated. However, the data did suggest that for oligometastatic “low-risk” disease (fewer than 5 bone metastases, and Gleason ≤ 8) the survival curves began to separate at the 2-year mark.34
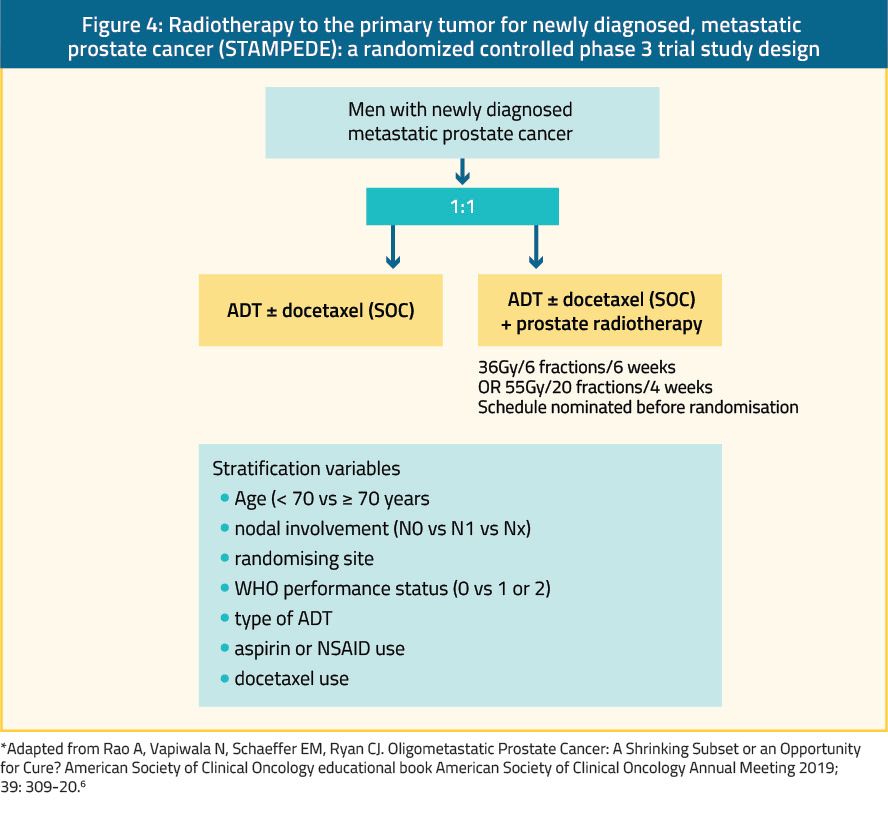
The second trial was the STAMPEDE trial, which is an ongoing multicenter, multi-arm, randomized controlled trial at 117 centers in the UK and Switzerland35 (Figure 4). Arm H of this trial enrolled 2,061 patients with newly diagnosed mHSPC with no prior therapies and randomly assigned them 1:1 to standard of care (ADT with or without docetaxel) vs. standard of care plus RT (RT group; RT dose was 55 Gy in 20 fractions or 36 Gy in 6 fractions).35 High metastatic burden was defined as per the CHAARTED trial definition of >=4 or more bone metastases with one or more outside the vertebral bodies or pelvis, or visceral metastases, or both.36 Approximately 42% of patients in each group had low-burden metastatic disease. In contrast to the HORRAD trial, this trial showed that RT improved the failure-free survival (FFS) compared with standard of care alone (HR 0.76; 95% CI, 0.68–0.84; p<0.0001), but OS was not improved (HR 0.92; 95% CI, 0.80–1.06; p =0.266).35 Importantly, in a prespecified subgroup analysis of patients with low-burden metastatic disease (819 patients) RT was shown to improve FFS (HR 0.59; 95% CI, 0.49–0.72; p<0.0001) and 3-year OS as well (81% vs. 73%; HR 0.68; 95% CI, 0.52–0.90; p=0.007).35 On the other hand, those with a high-burden metastatic disease did not have the same benefit as RT in terms of FFS or OS.35
The STOPCAP meta-analysis analyzed the results of the HORRAD, STAMPEDE trials and the ongoing PEACE-1 study (Figure 5).37 The PEACE-1 is a four-arm study randomizing men with mHSPC to ADT plus docetaxel and treatment with or without abiraterone and then randomizes them to receive radiotherapy directed at the prostate or no local therapy (NCT01957436). In this meta-analysis, radiotherapy did not clearly improve survival or PFS in unselected mHSPC men. However, stratification by the metastatic burden resulted in a clear difference in effect with an absolute improvement of 7% in three-year survival in men who had four or fewer bone metastases.37 The effect of RT on survival did not vary by other patient or disease characteristics. RT improved the three-year biochemical progression and FFS by approximately 10% in unselected men, but the size effect varied by metastatic burden.37

Metastases-directed therapy
Only a few studies have investigated a more aggressive approach using combined local therapy to the primary tumor and metastasis-directed therapy (MDT) in the de novo oligometastatic setting. Most data have been in the form of stereotactic body radiation therapy (SBRT), which is defined as high-dose radiotherapy given in few fractions directed at extracranial targets.38 The available retrospective-derived data are heterogeneous,39, 40 but suggest that MDT with RT can provide local control, and MDT RT-associated toxicities are minor. Many small retrospective reports have shown improved PFS and OS in the recurrent or de novo oligometastatic settings.41, 42 The first prospective multicenter randomized phase 2 trial supporting the role of MDT was the STOMP trial, published in 2018 by Ost et al.43 This trial recruited patients with asymptomatic oligometastatic HSPC who were randomized 1:1 to either surveillance or MDT of all detected lesions (surgery or SBRT). The 62 analyzed patients had oligorecurrent PCa with less than three extracranial metastases demonstrated by choline PET/CT. The primary endpoint was ADT-free survival, and ADT was started for symptomatic or local progression or upon the development of more than three metastases. After a median follow-up of 3 years, the median ADT-free survival was 21 months in the MDT arm compared with 13 months in the control group (p=0.11). The patients with PSA doubling times ≤3 months experienced a more significant benefit with MDT than with surveillance (HR 0.14 vs 0.44; P interaction = 0.01). Despite results highlighting the potential of MDT in delaying initiation of systemic therapy,44 no statistically significant improvement in the one-year quality of life was shown. Additionally, one of the limitations of the trial’s design is the nonstandard control arm, given the known OS benefit from immediate versus delayed ADT for men with biochemically recurrent PCa.45 Nevertheless, this trial supports the ongoing investigation of local ablative therapy for oligorecurrent PCa.43There have been several retrospective studies supporting surgical resection and RT of distant metastases including retroperitoneal lymph node dissection in patients with nodal-only recurrences of PCa.46, 47 The POPSTAR trial, was a single-arm, prospective clinical trial that assessed the safety and feasibility of SBRT (20 Gy, single fraction per site) to all metastatic sites in oligometastatic patients (3 or fewer sites of nodal or bony metastases as detected by NaF PET scan).48 A total of thirty-three patients received SBRT to 50 oligometastatic sites, (feasibility rate, 97%; 95% CI, 84%– 100%). In 22 mHSPC patients, freedom from ADT treatment at 24 months was 48% (95% CI, 31%–75%). The data from this small feasibility study support the use of SBRT to distant nodal and bony metastatic lesions with potential in delaying time to systemic therapy.48
Aside from the STOMP trial that included only six surgically-treated patients with MDT,43 there are currently no available prospective data evaluating surgical MDT. Most retrospective studies have specifically evaluated salvage lymphadenectomy in men with image-confirmed nodal recurrence following RP.49, 50 These showed that most patients experienced a decrease in PSA following the salvage procedure, with 80% of the men reaching a PSA nadir of less than 0.2 ng/ml and more than half the men being without radiographic recurrence at five years.50 The largest retrospective study assessing 654 men with nodal recurrent disease showed that ¾ of the men remained free of recurrence at one year following lymphadenectomy, with a median time to recurrence of 3 years.51
Ongoing and Future trials
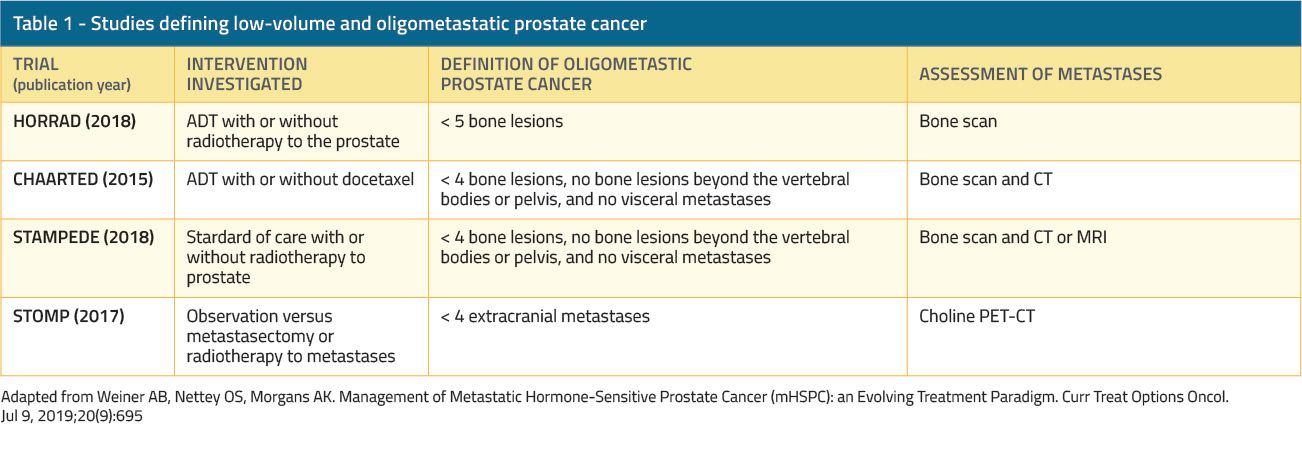
Table 1 shows the many current ongoing trials assessing treatment option for oligometastatic disease. These trials will address a substantial gap in the literature and provide much needed clinical guidance. Most protocols are nonrandomized phase 1 and 2 studies, and only 3 of 21 (14%) are randomized phase 2 and 3 or phase 3 protocols. This suggests that we are destined to wait a long time for definitive randomized phase 3 evidence powered to detect OS difference in this unique setting.8 The current most anticipated trial is the randomized phase 3 protocol sponsored by the US-based Southwest Oncology Group (S1802)52 (Figure 6). This trial plans to enroll 1273 patients with de novo oligometastatic and non-oligometastatic disease who are assigned to receive either standard systemic therapy or standard systemic therapy plus RP or RT to the prostate. This is the largest trial in this unique space and will allow all patients with the oligometastatic disease to receive MDT to ≤4 sites before randomization.52
Another anticipated trial is the randomized phase 2 ORIOLE trial, initiated in April 2016 and sponsored by the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins.53, 54 The inclusion criteria require ≤3 asymptomatic metastases measuring ≤5.0 cm in greatest dimension or <250 cm3 in volume that developed within 6 months before enrollment with a PSA doubling time <15 months and an Eastern Cooperative Oncology Group performance status ≤2. Patients are randomized in a 2:1 fashion to receive SBRT in 1 to 5 fractions to all metastases vs. observation. Patients who are randomized to SBRT will also undergo an investigational PSMA-based PET/CT.55 The results will be compared with a conventional bone scan and CT imaging to characterize the potential benefits of using this advanced functional imaging technique for the initial detection of oligorecurrent PCa and subsequent progression after MDT.
Other important prospective trials examining the role of treatment to the primary tumor in metastatic disease that should be mentioned include:
- The TROMBONE feasibility trial (ISRCTN15704862). This is a randomized phase II trial assessing the feasibility of RP in men with bone-only oligometastatic disease treated in the UK. It has completed the planned enrollment of 50 patients and results are expected soon.56
- The multicentric prospective Local treatment of Metastatic Prostate cancer (LoMP) trial has been investigating the role of cytoreductive RP in addition to the standard of care for mHSPC patients. The trial will assess the time to development of mCRPC (NCT02138721)
- The larger (predicted accrual ~ 452 men) German-led g-RAMMP trial which will investigate the effect of RP with extended lymphadenectomy on CSS, time to CRPC, time to progression and quality of life in patients with a limited bone mHSPC (NCT02454543).
- The PEACE-1 trial is a four-arm study randomizing men with mHSPC to ADT plus docetaxel to treatment with or without abiraterone and then randomizes them to receive RT directed at the prostate or no local therapy (NCT01957436). Radiation doses in this trial will be 74 Gy given in 37 fractions. The authors plan to enroll nearly 1200 men with final analyses set for 2030.
- A North American trial (NCT01751438) will assess PFS in 180 men receiving 6 months of best systemic therapy and then being randomized to receive either local therapy to the prostate or no local treatment.
Conclusions
Current data suggest that it is reasonable to offer oligometastatic patients, with newly diagnosed mHSPC the option of RT to the primary tumor.6 Data is still being collected whether surgery has an equal role in this setting. There is no doubt that the continued assessment of genomic and clinicopathologic characteristics of these patients is required to further refine the subset of patients that are most likely to benefit from this treatment strategy.6Ongoing trials assessing MDT may also shift the landscape, especially if these studies support a survival benefit associated with MDT. The ongoing work assessing advanced PET imaging and other novel imaging modalities will undoubtedly impact the mHSPC landscape as more patients with low volume metastatic disease / oligometastatic disease will be identified. Eventually, the goal should be to improve the survival and quality of life outcomes of oligometastatic mHSPC patients and perhaps even entertain the possibility of a cure.

Written by: Hanan Goldberg, MD, Department of Urology, SUNY Upstate Medical University, Syracuse, New York
Published Date: December 10th, 2019


