The rapid spread of Coronavirus Disease 2019 (COVID-19), caused by the betacoronavirus SARS-CoV-2, throughout the world has had dramatic effects on healthcare systems with impacts far beyond the patients actually infected with COVID-19.
Patients who manifest severe forms of COVID-19 requiring respiratory support typically require this for prolonged durations, with a mean of 13 days of respiratory support reported by the China Medical Treatment Expert Group for COVID-19.1 This lengthy requirement for ventilator support and ICU resources, exacerbated by relatively little excess health system capacity to accommodate epidemics, means that healthcare systems can (and have in the case of many hospitals in Italy) become overwhelmed relatively quickly. In an effort to conserve hospital resources, the American College of Surgeons on March 13th recommended that health systems, hospitals, and surgeons should attempt to minimize, postpone, or outright cancel electively scheduled operations.2 This was done with the primary goal to immediately decrease the use of items essential for the care of patients with COVID-19 including ICU beds, ventilators, personal protective equipment, and terminal cleaning supplies. On March 17th, the American College of Surgeon then provided further guidance on the triage of non-emergent surgeries, including an aggregate assessment of the risk incurred from surgical delays of six to eight weeks or more as compared to the risk (both to the patient and the healthcare system) of proceeding with the operation.3 In the UK, all non-urgent elective surgical procedures have been put on hold for three months to use all of those clinical resources to care for patients with COVID-19.
Most bodies, including the American College of Surgeons, have recommended proceeding with most cancer surgeries. Thus, clinicians and patients must carefully weigh the benefit of proceeding with cancer treatment as scheduled, the risks of COVID-19 to the individual patient, to health care workers caring for patients potentially infected with COVID-19, and the need to conserve health care resources. A severe SARS-CoV-2 phenotype is seen more commonly in men and older, more comorbid patients.4 These characteristics are common in many patients with urologic malignancies, particularly those with bladder cancer. Baseline characteristics among 1,591 patients admitted to the ICU in the Lombardy Region, Italy showed that the median age was 63 years (IQR 56-70), 82% were male, 68% had ≥1 comorbidity, 88% required ventilator support, and the mortality rate was 26%, with a large proportion requiring ongoing ICU level care at the time of data cut-off.5 Work from China demonstrated that patients with cancer had a higher incidence of COVID-19 infection than expected in the general population and had a more severe manifestation of the disease with a significantly higher proportion requiring invasive ventilation in the ICU or death.6 Thus, considering differences in the natural history of different cancers may meaningfully change this balance of risks and benefits.
In the urologic literature, the effect of delays in surgical intervention has been most thoroughly explored in muscle-invasive bladder cancer and prostate cancer. In bladder cancer, these studies have predominately assessed the association between time from transurethral resection of bladder tumor (TURBT) to radical cystectomy. At least nineteen studies have been published assessing this research question. Published October 23, 2019, in European Urology Oncology, Dr. Russell and colleagues provide a contemporary systematic review and meta-analysis of these data.7 The authors undertook a systematic review of Medline®, Embase®, and Ovid® for randomized trials and observational studies assessing the association between delay in treatment and survival (overall or bladder cancer-specific) for patients with bladder cancer. Among 399 identified articles, the authors included 19 studies in systematic review and 10 in meta-analysis. Utilizing the Risk of Bias in Non-randomised studies – of Interventions (ROBINS-I) and the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) criteria, the authors assessed that most studies were of good quality with low or moderate risk of bias. To account for this, the authors performed “leave out one” sensitivity analyses.
Perhaps the most striking conclusion of this systematic review is the considerable heterogeneity in the source literature, including in methodology. There was considerable variation in the nature of the delay investigated: from the diagnosis of bladder cancer to radical cystectomy (10 studies), from TURBT to radical cystectomy (seven studies), from first clinic visit to radical cystectomy (or radiotherapy; one study), from referral to first treatment (one study), and from neoadjuvant chemotherapy to radical cystectomy (four studies). Additionally, the delay interval was operationalized inconsistently across studies with some using time as a continuous variable, some utilizing splines to model a non-linear relationship, and many utilizing a categorical approach with a variety of thresholds.
Among these studies, a number assessed reasons for delay. Identified reasons for delay included scheduling, seeking multiple medical opinions, social issues, misdiagnosis, and patient comorbidity.
Assessing the delay between bladder cancer and survival, four of nine studies found a significant association between delay from diagnosis to radical cystectomy and survival, with a number concluding that tumor stage was a potential confounder in this relationship. Russell and colleagues meta-analyzed three studies with suitable data and found an increased risk of death for patients with significant delays between diagnosis and radical cystectomy (hazard ratio [HR] 1.34, 95% confidence interval [CI] 1.18-1.53; I2=0%).7
Operationalizing delay as the interval between TURBT and radical cystectomy, four of six studies found an association between this time and survival; a meta-analysis of five studies with suitable data for pooling found a borderline non-significant increased risk of overall mortality (odds ratio 1.18, 95% confident interval 0.99-1.41), with significant between study heterogeneity (I2=73%).7 Utilizing a cubic spline to model the non-linear relationship, Kulkarni and colleagues found that the risk of death began to rise beginning at 40 days between TURBT and radical cystectomy.8
An additional five studies assess the association between the time duration between the completion of neoadjuvant chemotherapy and radical cystectomy and survival. Two demonstrated that prolonged durations between neoadjuvant chemotherapy and radical cystectomy was associated with adverse survival outcomes and an additional one demonstrated upstaging was associated with delays. Boeri et al. found that patients who had greater than 10 weeks between the last cycle of neoadjuvant chemotherapy and radical cystectomy had significantly lower cancer-specific and overall survival.9 Chu et al. found similar results10 while three other analyses failed to support these results. A meta-analysis of three studies with data suitable for pooling failed to demonstrate a significant association between delays from the end of neoadjuvant chemotherapy to radical cystectomy with survival (HR 1.04, 95% CI 0.93-1.16; I2=82%).7
Particularly relevant in the context of the COVID-19 pandemic, Audenet and colleagues found that delays to neoadjuvant chemotherapy of greater than eight weeks were associated with an increased risk of upstaging, while they found no harm in delays up to six months from diagnosis to radical cystectomy, assuming that neoadjuvant chemotherapy was administered in the meantime.11
While the meta-analysis of Russell and colleagues focused on patients with urothelial histology, recently Lin-Brande examined outcomes for patients with variant histology undergoing radical cystectomy.12 In this analysis, patients with variant histology had a similar time from diagnosis to radical cystectomy as those with urothelial histology. In this cohort, delays from diagnosis to radical cystectomy were associated with worse overall survival (HR 1.36, 95% CI 1.11-1.65 per month of delay) after adjusting for relevant clinicopathologic features. The authors then subsequently dichotomized surgical delays using thresholds of four-, eight-, and 12-weeks. On multivariable analysis, no difference in overall survival was apparent when “early” versus “late” was dichotomized at four weeks (hazard ratio 0.92, 95% CI 0.32-2.59) or eight weeks (HR 1.50, 95% CI 0.68-3.29) but significant differences were apparent when delayed surgery was defined as that beyond 12 weeks following diagnosis (HR 3.45, 95% CI 1.51-7.86).
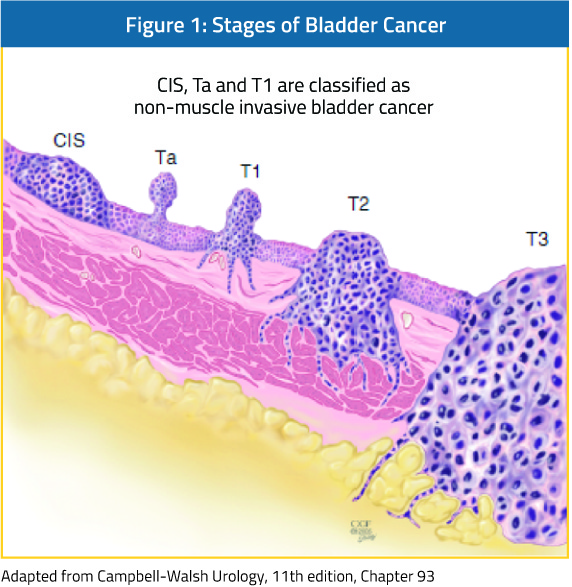
There is somewhat less evidence in patients with non-muscle invasive disease, with significant differences between patients with low-grade and high-grade disease. Low-grade non-muscle invasive bladder cancer has low cancer-specific mortality13 and there is no evidence of harm from delays in management.14-16
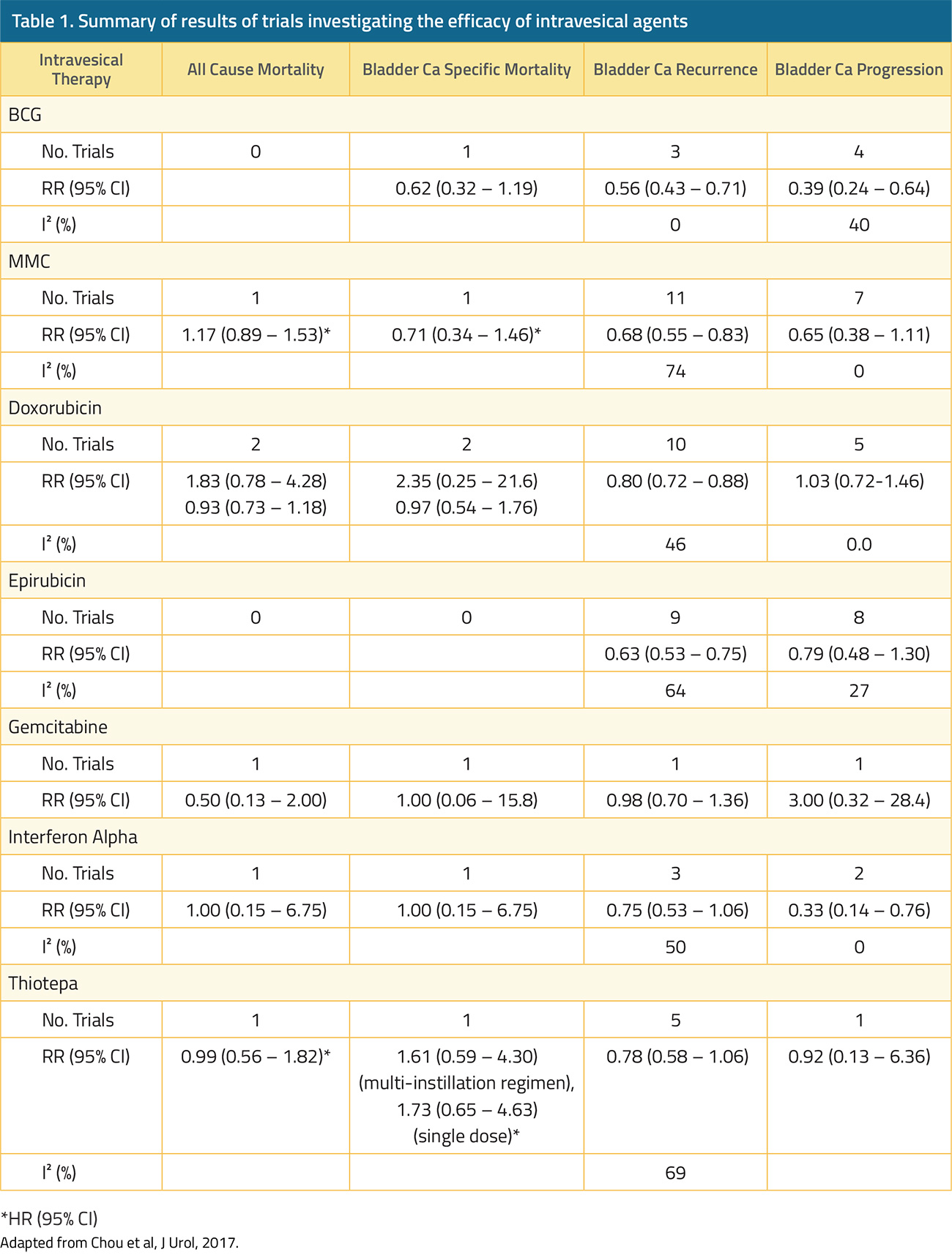
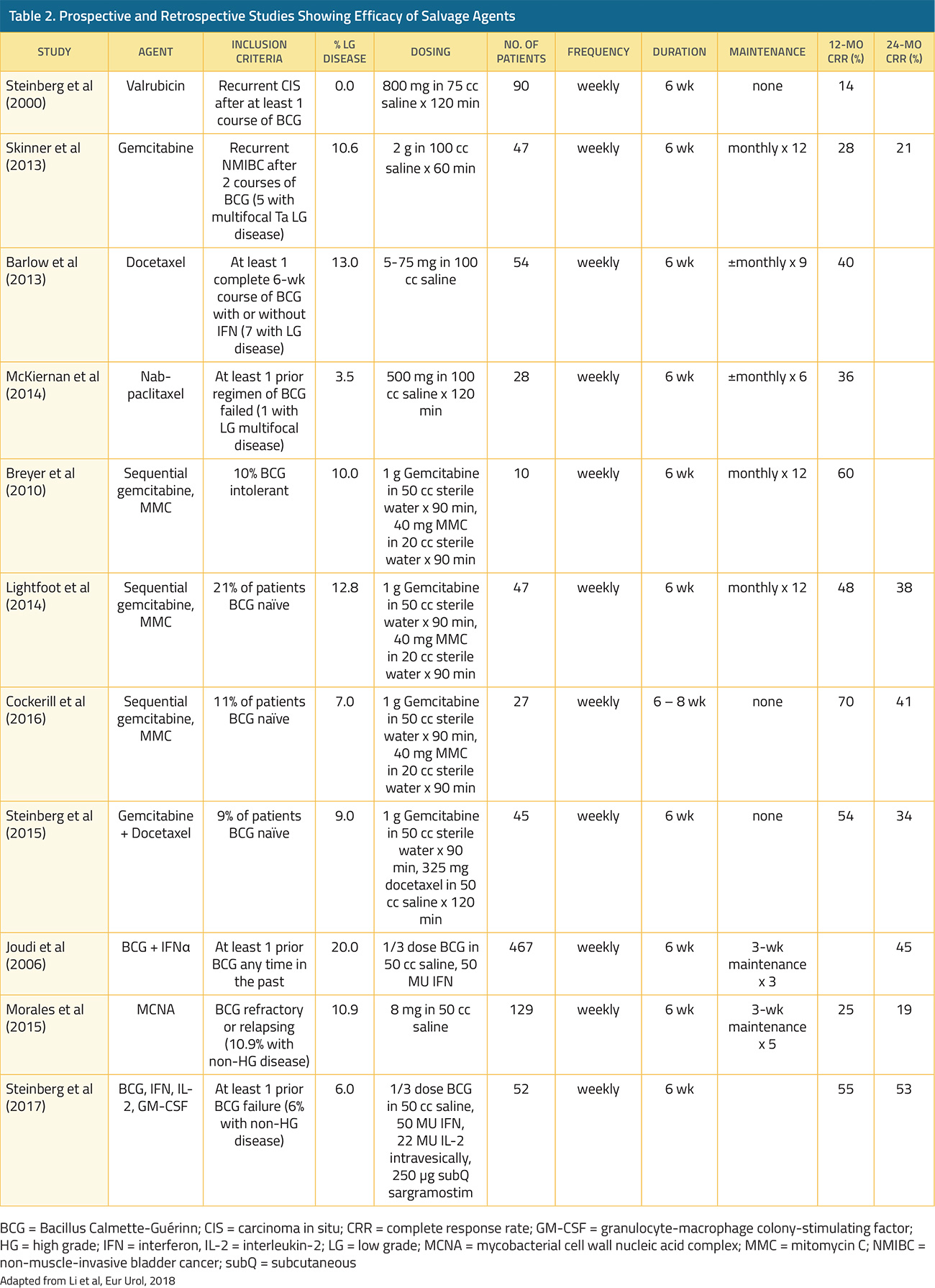
In contrast, for patients with high-grade non-muscle invasive disease, progression to muscle invasion/metastases occurs in 15-40% and 10-20% of patients may die from bladder cancer.17,18 In patients who underwent radical cystectomy for recurrent non-muscle invasive bladder cancer following Bacillus Calmette-Guerin (BCG) with or without further intravesical therapy, the delay caused by an additional (unsuccessful) course of intravesical therapy did not result in differences in five-year overall or cancer-specific survival despite a median delay of 1.7 years.19 In contrast, among patients with non-muscle invasive (cT1) micropapillary bladder cancer treated with upfront radical cystectomy or intravesical BCG, Willis et al. demonstrated significantly poorer survival outcomes for patients who underwent initial intravesical therapy.20
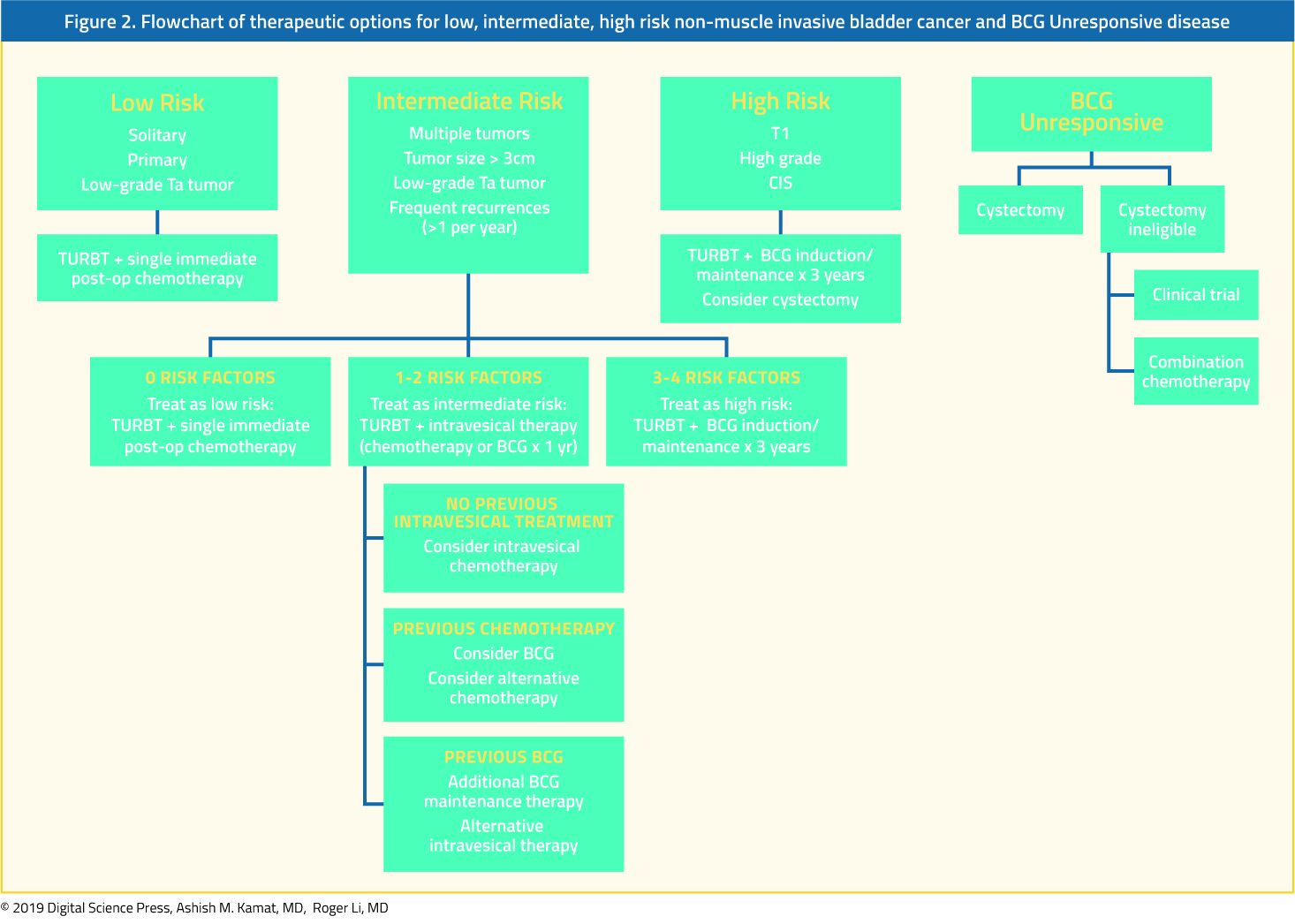
In the absence of data, guidance on the care of patients with high-grade non-muscle invasive bladder cancer has relied upon expert guidance. A collaborative review pre-published in European Urology suggested that these patients should receive induction BCG and at least one course of maintenance therapy as the first-line treatment. The authors recommended that re-resection should be continued for patients with pT1 disease while this may be omitted for those with pTa and muscle in the initial resection.
The data regarding delays to radical cystectomy demonstrate considerable heterogeneity with mixed results. This is in large part due to varying definitions of a delay, despite the threshold of 12 weeks advocated in EAU guidelines.21 However, in aggregate, these data suggest that prolonged delays (likely 90 days although potentially as short as 40 days) between bladder cancer diagnosis or TURBT and radical cystectomy are associated with worse survival. However, the data are mixed and pooled results demonstrate considerable heterogeneity. Further, when neoadjuvant chemotherapy is employed, delays to radical cystectomy no longer appear to be significant. Finally, an analysis of funnel plots indicates that there is a publication bias towards studies which demonstrate worse survival associated with delays suggesting that this finding may be exaggerated in the literature.7
A recent multi-institutional analysis from Campi and colleagues assessed the proportion of patients undergoing urologic oncology surgery who had “nondeferrable” indications for treatment.22 The authors identified 2387 patients undergoing radical cystectomy, radical nephroureterectomy, nephrectomy, and radical prostatectomy in the past 12 months at San Luigi Hospital in Turin, San Raffaele Hospital in Milan, and Careggi Hospital in Florence. Assessing patients undergoing radical cystectomy, the authors considered all cases “nondeferrable”. Radical cystectomy comprised 36.2% of all so-called high-priority or nondeferrable cases. They also noted that a large proportion of these patients (50%) are at elevated perioperative risk (defined at American Society of Anesthesiologists score ≥ 3).
In the context of non-muscle invasive disease, delays appear to be significantly more likely to cause harm in patients with high-grade than low-grade disease. However, the evidence base in non-muscle invasive disease is much weaker than for muscle-invasive bladder cancer.
However, when considering delays in performing TURBT, it is not always apparent whether the patient has non-muscle invasive or muscle-invasive disease. Wallace and colleagues examined delays throughout the trajectory of patients with a new diagnosis of urothelial carcinoma, including a preponderance of non-muscle invasive disease (pTa = 51% and pT1 = 22%).23 The authors divided delays in the evaluation of these patients into three: from initial symptom onset to general practitioner presentation (patient-derived delay), from general practitioner presentation to hospital referral (general practitioner-derived delay), and from hospital referral to TURBT (hospital-derived delay). Shorter delays from initial symptom onset to general practitioner presentation were associated with lower tumor stage and improved five-year survival. In this analysis, the authors dichotomized this patient-derived delay at 14 days. Notably, patients with shorter general practitioner-derived delay had worse survival, likely reflecting a selection bias in which patients with particularly worrisome presentations received expedited care. Finally, hospital-derived delays were not significantly associated with survival, whether adjusted for tumor stage or not. When considered in aggregate, total delays from initial symptom onset to TURBT were not significantly associated with survival.
During the COVID-19 pandemic, there will be regional variations in the risk of infection and availability of resources, both with regards to medical treatment and operating room availability. Expert recommendations from the pre-published paper in European Urology suggest it is reasonable that patients with high-grade NMIBC should undergo induction BCG and maintenance therapy if possible. For high-risk NMIBC, radical cystectomy should still be offered if the resources are feasible and the patient’s comorbidity profile does not place them at higher post-operative COVID-19 risk. Based on the available literature, delays in radical cystectomy of up to three months may be safe for muscle-invasive bladder cancer. However, clinicians should prioritize high-grade bladder cancer (NMIBC and muscle-invasive) over other urologic oncology procedures during COVID-19 restrictions.
Written by: Zachary Klaassen, MD, MSc, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Atlanta, Georgia
Published Date: April 20th, 2020