Introduction
Since 2015, the metastatic hormone sensitive prostate cancer (mHSPC) disease space now has several options of doublet and triplet therapy, using ADT as the backbone of treatment, leading to an overall survival (OS) advantage versus ADT alone. Thus, this has changed the standard of care for treatment intensification for these men. The incidence of metastatic prostate cancer at diagnosis ranges from ~5-50%, with vast geographic differences. Such patients are defined as having de novo or synchronous mHSPC. Additionally, some men who are initially diagnosed with non-metastatic disease will have progression to metastasis prior to development of castration resistance, also known as metachronous mHSPC.1This distinction between synchronous and metachronous mHSPC is of utmost clinical importance given differences in prognosis,2 genomic mutational profiles,3,4 and recommended systemic treatment options between these two groups.3 Both groups can be further stratified by volume of metastatic disease, most commonly by using the CHAARTED high-volume criteria as follows: presence of visceral metastases or ≥4 bone lesions with ≥1 beyond the vertebral bodies and pelvis.5 As such four distinct subgroups become clinically relevant (median OS per CHAARTED and GETUG-15 among men receiving ADT alone, ie. the control groups in these trials):
- Metachronous and low volume: ~8 years
- Metachronous and high volume: 4.5 years
- Synchronous and low volume: 4.5 years
- Synchronous and high volume: 3 years
Given that the results of the key phase 3 mHSPC randomized clinical trials in the intention to treat population are discussed in another Center of Excellence article, the following article will take on more a nuanced approach assessing outcomes of these trials focusing specifically on timing of metastatic disease (de novo versus metachronous) and volume of disease (high versus low). Additionally, we will address several specific treatment considerations (ie. metastasis directed therapy [MDT] and prostate radiotherapy) for those with very low, or oligometastatic, mHSPC.
ADT + Docetaxel
The CHAARTED trial was initially published in 2015 and an updated analysis was published in 2018 evaluating the survival benefit of docetaxel addition to ADT by volume status.6 This trial was enriched for high volume disease (64.9% of total cohort) and 72.8% of patients presented with de novo disease. This updated analysis demonstrated that the benefit of docetaxel addition is restricted to patients with high volume disease (median OS 51.2 months for docetaxel + ADT versus 34.4 months for ADT alone; HR 0.63, 95% CI 0.50 to 0.79) but not low volume disease (median OS 63.5 months for docetaxel + ADT versus not reached for ADT alone; HR: 1.04, 95% CI 0.70 to 1.55):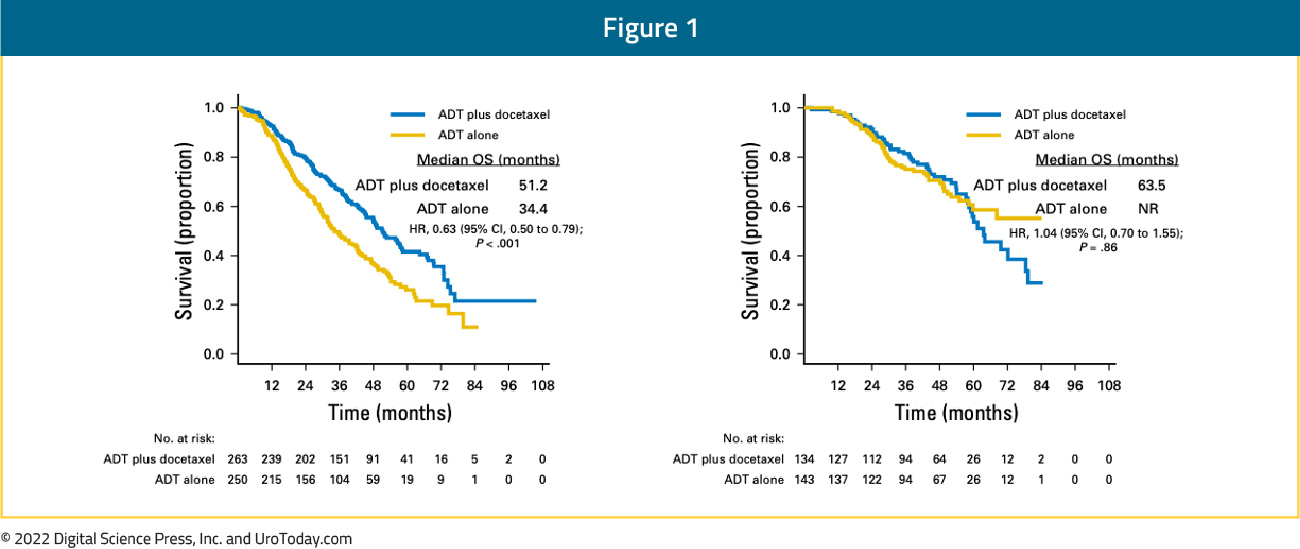
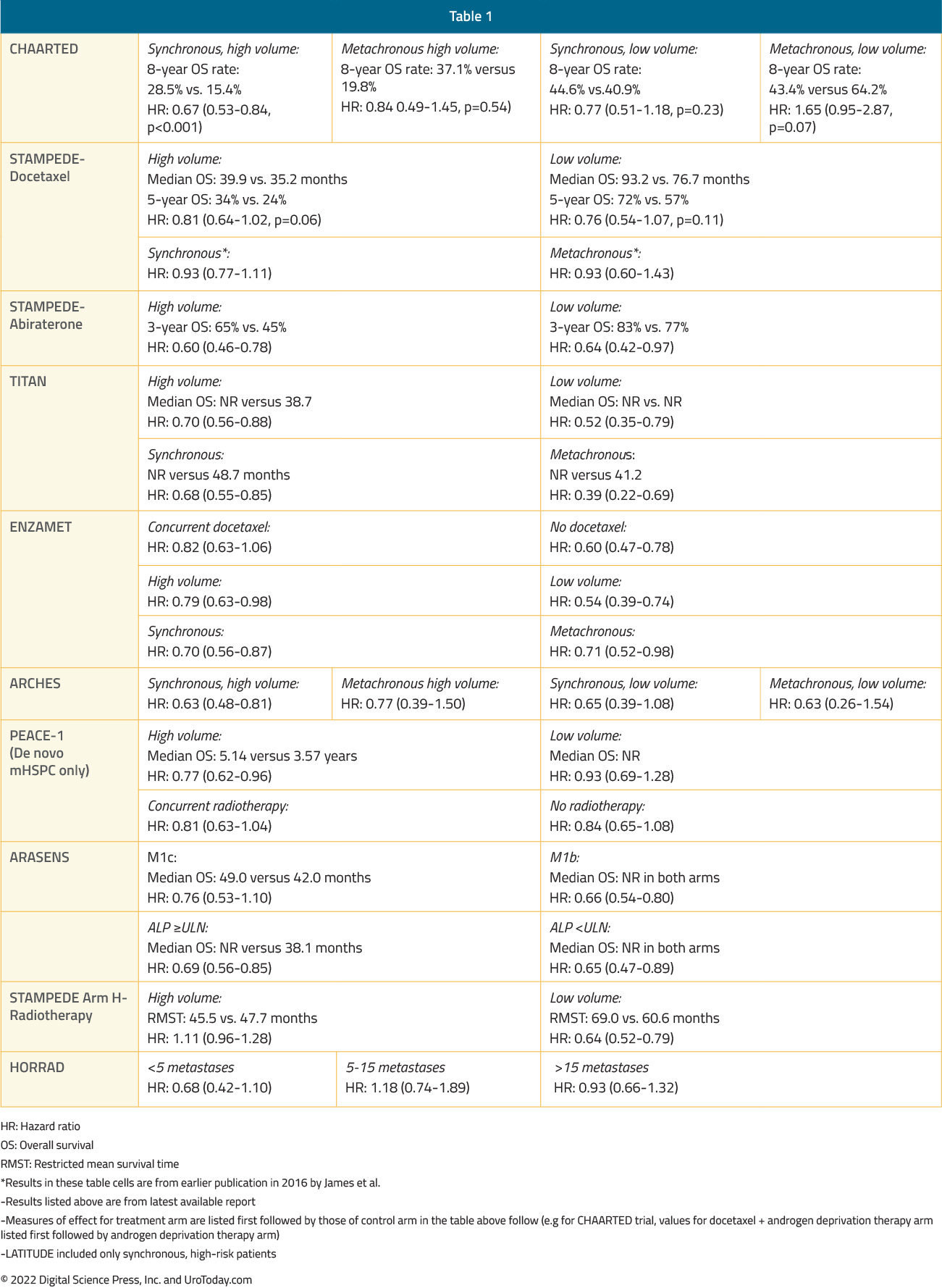
Patients with high volume disease appeared to derive a benefit from docetaxel addition irrespective of whether they present with de novo metastatic disease (median OS 48.0 versus 33.1 months; HR 0.63, 95% CI 0.59 to 0.81) or metastatic recurrence following prior local therapy (median OS 66.9 versus 51.7 months; HR 0.72, 95% CI 0.36 to 1.46). There was no benefit seen in the low volume groups, irrespective of whether presenting with de novo or recurrent mHSPC.6 The most recent update at ASCO 2022 presented the 8-year OS data for patients from the CHAARTED trial. This update confirmed an OS benefit for docetaxel addition in the high-volume cohort, however, it also demonstrated a mortality benefit for synchronous low-volume mHSPC patients with OS rates of 44.6% versus 40.9% (HR 0.77, 95% CI 0.51 to 1.18), but not metachronous low-volume patients (43.4% versus 64.2%; HR 1.65, 95% CI 0.95 to 2.87):

The GETUG-15 trial included 70.6% patients with de novo mHSPC, with only 50% of patients considered high volume per the CHAARTED criteria (compared to 64.9% in CHAARTED). This may in part explain the negative OS outcome seen in GETUG-15. Long-term follow-up from GETUG7 and combined analysis from GETUG-15 and CHAARTED8 demonstrated benefit for docetaxel addition in the high volume mHSPC, but not those with low-volume disease.
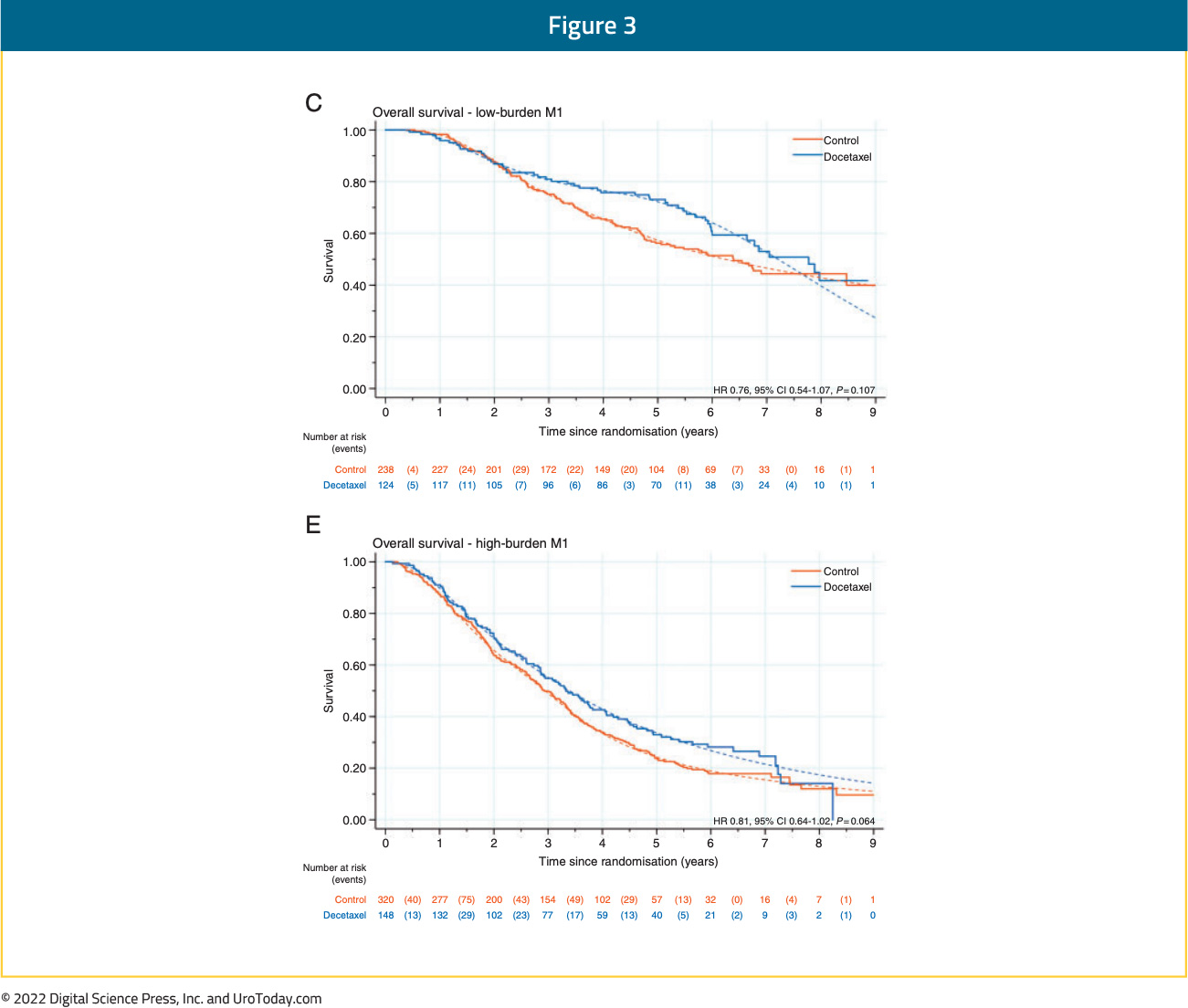
Updated results from the STAMPEDE trial published in 2019 with a median follow up of 78.2 months retrospectively evaluated imaging results for patients with available baseline staging scans (76%). This demonstrated consistent OS benefits for docetaxel in both the low and high-volume cohorts (CHAARTED criteria). In low volume patients, median OS improved from 76.7 months to 93.2 months with docetaxel (HR 0.76, 95% CI 0.54 to 1.07) compared to 39.9 months from 35.2 months in the high-volume patients (HR 0.81, 95% CI 0.64 to 1.02) (interaction by metastatic burden p=0.827).9
At ASCO 2022, the results of a STOPCAP M1 collaborative meta-analysis of individual patient data from GETUG-15, STAMPEDE, and CHAARTED was presented. This meta-analysis included all 2,261 randomized patients, with median follow-up of 6 years. There were clear benefits for docetaxel on OS (HR 0.79, 95% CI 0.70 to 0.88), progression-free survival (PFS) (HR 0.70, 95% CI 0.63 to 0.77) and failure-free survival (FFS) (HR 0.64, 95% CI 0.58 to 0.71) in the overall pooled cohort. With evidence of non-proportional hazards, the estimated 5-year absolute differences were: OS 11% (95% CI 6 to 15%), PFS 9% (95% CI 5 to 13%) and FFS 9% (95% CI 6 to 12%).
Notably, the relative effect of docetaxel on PFS differed by volume of metastases (interaction p=0.027; high volume HR 0.60, 95% CI 0.52 to 0.68; low volume HR 0.78, 95% CI 0.64 to 0.94) and timing of metastatic disease (interaction p=0.077; synchronous HR 0.67, 95% CI 0.60 to 0.75; metachronous HR 0.89, 95% CI 0.67 to 1.18). OS results were similar. When metastatic disease volume and timing were combined, docetaxel appeared to improve PFS and OS for all men, except those with low volume, metachronous disease, though there is a clear dose-response relationship with a diminishing benefit to docetaxel chemotherapy from high-volume synchronous disease, to high-volume metachronous disease, to low-volume synchronous disease, and finally to low-volume, metachronous disease:
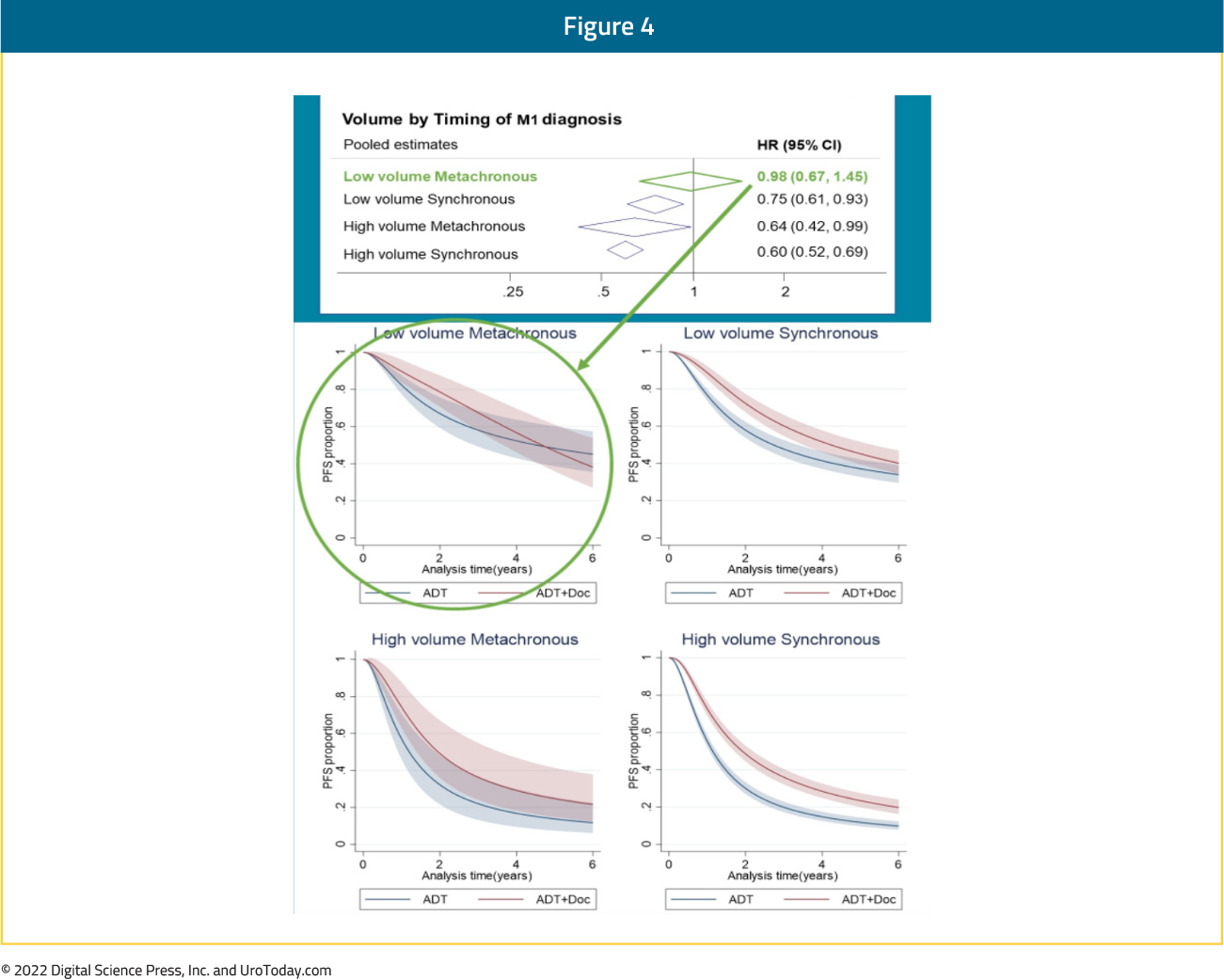
Taken together, it appears that docetaxel addition has a clear OS benefit in patients with high volume disease, irrespective of timing of disease (synchronous or metachronous). Patients with synchronous low-volume disease may have a modest benefit with docetaxel addition though those with low volume, metachronous (i.e. recurrent) mHSPC do not benefit from docetaxel addition to ADT.
ADT + Abiraterone/Prednisone
The LATITUDE trial published in 2017 included only patients with de novo, high risk prostate cancer, leading to the LATITUDE high risk criteria (as opposed to high versus low volume), defined as patients with two or more of the following characteristics: (i) Gleason Score ≥8, (ii) presence of ≥3 lesions on bone scan, and (iii) presence of measurable visceral lesions. These risk criteria were applied in a post hoc subgroup analysis of the 2017 STAMPEDE “abiraterone comparison”.10Among 990 patients, 901 patients with available data were included. LATITUDE high-risk disease was present in 52% while the remainder had so-called low-risk disease. Overall survival benefits with ADT + abiraterone/prednisone addition were seen in both the low (HR 0.66, 95% CI 0.44 to 0.98) and high-risk groups (HR 0.54, 95% CI 0.41 to 0.70). The heterogeneity of treatment effect between high- and low-risk groups was not statistically significant (p-interaction = 0.39). When the CHAARTED criteria were applied in this same cohort, consistent OS benefits were again seen in both the low- (HR 0.64, 95% CI 0.42 to 0.97) and high-volume groups (HR 0.60, 95% CI 0.46 to 0.78).
ADT + Apalutamide vs ADT
In the TITAN trial, 81% of patients presented with de novo mHSPC and 62.7% of patients had CHAARTED high volume disease.11 A subgroup analysis from TITAN demonstrated that addition of apalutamide maintained OS benefits irrespective of:Disease volume
- High: HR: 0.70 (95% CI: 0.56-0.88)
- Low: HR: 0.52 (95% CI: 0.35-0.79)
- Synchronous: HR: 0.68 (95% CI: 0.55-0.85)
- Metachronous: HR: 0.39 (95% CI: 0.22-0.69)
ADT + Enzalutamide vs ADT
In 2021, Sweeney et al. performed subgroup analyses of patients with metachronous mHSPC treated in the ENZAMET trial of enzalutamide. Of the 1,125 enrolled patients, 312 (28%) had known metachronous disease and 205 (66%) had low-volume disease at entry. For the metachronous mHSPC group overall, OS HR was 0.56 (95% CI 0.29 to 1.06) for enzalutamide addition. Interestingly, this OS benefit seemed to be driven mainly by benefits in the low-volume subgroup (HR 0.40, 95% CI 0.16 to 0.97) with no apparent benefit in the high volume metachronous subgroup (HR 0.86, 95% CI 0.33 to 2.22). These results may have been secondary to the high use of concurrent docetaxel in the high-volume subgroup (60% vs 15% in patients with low-volume disease), which may have diluted an OS benefit in the high volume subgroup.12Most recently at ASCO 2022, Dr. Ian Davis presented the most recent update of the ENZAMET trial. With regards to subgroup analyses, enzalutamide demonstrated consistent OS benefits across the following strata:
Planned early docetaxel (p-value for interaction=0.09)
- Yes: HR 0.82 (95% CI 0.63 to 1.06)
- No: HR 0.60 (95% CI 0.47 to 0.78)
- Low: HR 0.54 (95% CI 0.39 to 0.74)
- High: HR 0.79 (95% CI 0.63 to 0.98)
- Synchronous: HR 0.70 (95% CI 0.56 to 0.87)
- Metachronous: HR 0.71 (95% CI 0.52 to 0.98)
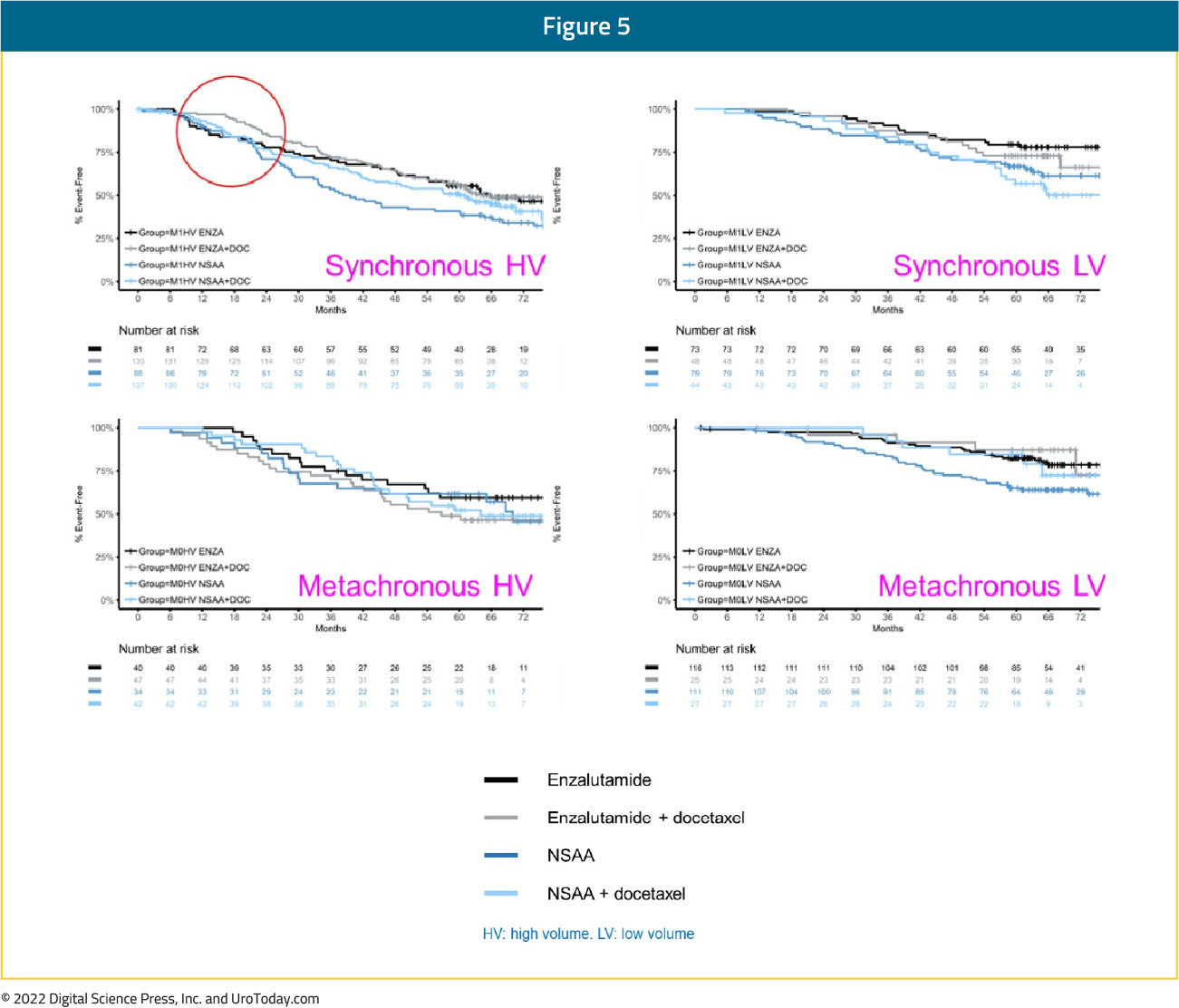
In the ARCHES trial, patients with predominately high-volume (63%) and de novo (66.7%) disease were randomized to enzalutamide + ADT or ADT alone. Notably, 17% of the cohort had previously received docetaxel.13 The most recent update of the trial was presented at ASCO GU 2022, noting that there were consistent benefits for the use of enzalutamide in addition to ADT in all disease volume and M0/M1 populations:
- Synchronous, high volume: HR 0.63 (95% CI 0.48 to 0.81)
- Synchronous, low volume: HR 0.65 (95% CI 0.39 to 1.08)
- Metachronous, high volume: HR 0.77 (95% CI 0.39 to 1.50)
- Metachronous, low volume: HR 0.63 (95% CI: 0.26 to 1.54)
ADT + Docetaxel + Abiraterone
Initially presented at ASCO 2021 and subsequently published in the Lancet in 2022,22 the PEACE-1 trial employed a 2x2 design to assess, (separately and combined) the impact of the addition of abiraterone + prednisone + ADT and radiation therapy to standard of care therapy in men with de novo mHSPC. Among patients with high volume disease, the addition of abiraterone + prednisone + ADT to standard of care resulted in a 53% improvement in rPFS with median rPFS of 1.6 years on the standard of care arm and 4.1 years on the standard of care plus abiraterone + prednisone + ADT arm (HR 0.47, 95% CI 0.36 to 0.60). The addition of abiraterone + prednisone + ADT to standard of care in patients with low volume disease resulted in a 42% improvement in rPFS with median rPFS of 2.7 years on the standard of care arm versus not yet reached on the standard of care plus abiraterone + prednisone + ADT arm (HR 0.58, 95% CI 0.39-0.87). With regards to OS, this effect was seen across subgroups, including those with high volume disease (HR 0.72, 95% CI 0.55 to 0.95) and low volume disease (HR 0.83, 95% CI 0.50 to 1.38; interaction P-value 0.64). Notably, the OS data is immature for the low volume patients due to a small number of events.ADT + Docetaxel + Darolutamide
The ARASENS trial evaluating addition of darolutamide to standard of care therapy consisting of ADT + docetaxel was presented at ASCO GU 2022 and concurrently published in The New England Journal of Medicine.14 Metastatic burden classification by CHAARTED criteria was not available in this trial, however disease stratification by TNM metastatic burden (M1b versus M1c) demonstrated consistent benefits for darolutamide addition to ADT and docetaxel. In the M1b subgroup (with bony metastases), HR for OS was 0.66 (95% CI 0.54 to 0.80). In the M1c group (with visceral metastases), HR for OS was 0.76 (95% CI 0.53 to 1.10), with median OS of 49.0 months in the darolutamide arm and 42.0 months in the placebo arm.The following table summarizes OS outcomes by CHAARTED disease volume criteria and presentation (synchronous vs metachronous) among men with mHSPC:
Specific Considerations for Low Volume mHSPC Patients
Beyond systemic treatment intensification, there is a role for therapy targeting either the primary (for patients with de novo disease) or metastatic sites in certain patient groups.Prostate radiotherapy: STAMPEDE (Arm H) was an open label, randomized controlled phase III trial of 2,061 men with de novo mHSPC randomized to standard of care + radiotherapy or standard of care. Subgroup analysis by metastatic volume (CHAARTED criteria) was planned a priori. Radiotherapy when stratified by metastatic burden showed an OS benefit in the low volume group (HR 0.68, 95% CI 0.52 to 0.90) with restricted mean survival time improved by 3.6 months from 45.4 to 49.1.15 Updated results of this trial were published June 2022 in PLoS Medicine.16 With a median follow up of 61.3 months, prostate radiotherapy continued to demonstrate OS benefits in patients with low metastatic burden (HR 0.64, 95% CI 0.52 to 0.79). No benefit was seen in patients with high metastatic burden (HR 1.11, 95% CI 0.96 to 1.28; interaction p=0.001).
HORRAD was a multicenter trial of 432 patients with previously untreated, de novo mHSPC men randomized in a 1:1 fashion to either ADT with external beam radiotherapy or ADT alone. No subgroup analyses by CHAARTED volume criteria was performed, but subgroup analysis by number of metastatic lesions suggested potential (albeit not statistically significant) OS benefit for radiotherapy in patients with <5 metastatic sites (HR 0.68, 95% CI 0.42 to 1.10).17
In 2019, a systematic review and meta-analysis of the STAMPEDE Arm H and HORRAD trials was performed by the STOPCAP collaboration. Pooled results of 2,126 men demonstrated no overall OS improvement (HR 0.92, 95% CI 0.81 to 1.04) or PFS (HR 0.94, 95% CI to 0.84-1.05). However, the effect of prostate radiotherapy on OS varied by metastatic burden (<5 versus ≥5 bone metastases: interaction HR 1.47, 95% CI 1.11 to 1.94, p=0.007). Furthermore, there was a 7% improvement in 3-yr survival in men with fewer than five bone metastases.18
Metastasis-Directed Therapy: In addition to systemic treatment intensification and local prostate-directed radiotherapy, treatment may be intensified by targeting local treatment to sites of metastatic disease. To date, there is no level one evidence supporting MDT in synchronous oligometastatic mHSPC disease space. Three randomized trials to date have evaluated MDT (stereotactic body radiotherapy or surgical metastasectomy) in patients with metachronous, oligometastatic mHSPC.
The STOMP trial was a multicenter, randomized phase II trial that prospectively evaluated the effects of MDT for patients with evidence of oligometastatic disease on choline PET/CT (up to three extracranial sites) who had received prior treatment with curative intent and had evidence of biochemical recurrence with testosterone >50 ng/ml (i.e. metachronous, oligometastatic mHSPC). Between 2012 and 2015, 62 patients were randomized 1:1 and MDT was either SBRT or metastasectomy. The primary endpoint was time to initiation of ADT (called ADT-free survival). ADT was initiated for symptoms, progression beyond three metastases, or local progression of known metastatic disease. Time to castration resistance was a secondary endpoint (called CRPC-free survival). The updated five-year results were presented at GU ASCO 2020. With a median follow up of 5.3 years, the five-year ADT-free survival was 8% in the surveillance arm compared to 34% for the MDT group (HR 0.57, 95% CI 0.38 to 0.84, log-rank p=0.06). No differences were seen between groups when stratified by nodal versus non-nodal metastases. Secondary endpoint of CRPC-free survival at 5 years was 53% in subjects under surveillance and 76% in those receiving MDT (HR 0.62, 80% CI 0.35 to 1.09).19
The ORIOLE trial was a randomized phase II trial of 54 men with metachronous, oligometastatic mHSPC (up to three sites). Metastatic sites were diagnosed via 18F-DCFPyL PET/CT. Between 2016 and 2018, patients were randomized in a 2:1 fashion to receive SABR or observation. The primary outcome was progression at 6 months, defined as serum PSA increase, progression detected by conventional imaging, symptomatic progression, ADT initiation for any reason, or death. Progression at six months occurred in 7 of 36 patients (19%) receiving SABR and 11 of 18 patients (61%) undergoing observation (p = 0.005). Treatment with SABR improved median PFS (not reached vs 5.8 months; HR 0.30, 95% CI 0.11 to 0.81). No toxic effects of grade 3 or greater were observed.20
SABR-COMET was a randomized, open-label phase II study of patients with oligometastatic disease (up to five sites) between February 2012 and August 2016. This trial was not restricted to patients with prostate cancer and also included lung, breast, and colorectal cancer patients. Of the 99 patients in this trial, 18 (18%) had prostate cancer. After stratifying by the number of metastases (1–3 vs 4–5), patients were randomized in a 1:2 fashion to receive either palliative standard of care alone or standard of care plus SABR. In an updated analysis published in 2020 (median follow up 51 months), the five-year OS rate was 17.7% (95% CI 6-34%) in the control arm and 42.3% in the SABR arm (95% CI 28-56%, stratified log-rank p=0.006). The corresponding median OS was 28 months and 50 months, respectively. There were no new grade 2-5 adverse events and no differences in QOL between the arms.21,22
Synthesizing the Timing of Metastasis and Volume of Disease Relationship
In clinical practice, the two faces of mHSPC may be very different. Dr. Chris Sweeney has lectured at length regarding the differences between de novo mHSPC (ie. a 55-year-old with no comorbidities and high volume de novo/synchronous metastatic disease) and metachronous mHSPC (ie. an 82-year-old with congestive heart failure and coronary artery disease with 2 bone metastases 10 years after prostatectomy). As highlighted above, when assessing the 8-year OS CHAARTED data, the addition of docetaxel to ADT shows a clear survival benefit for those with de novo high volume disease and those with metachronous high volume disease, a modest effect for those with synchronous low volume disease, and no benefit for those with metachronous low volume disease, men with biochemical relapse, and in the adjuvant setting. Alternatively, this is not the case for second generation anti-androgens: enzalutamide + ADT in ENZAMET showed improved PFS and OS among all subgroups (synchronous high-volume, synchronous low-volume, metachronous high-volume, and metachronous low-volume). This is also true in the ARCHES, TITAN, LATITUDE and STAMPEDE-abiraterone trials. Interestingly, subgroup analyses from ENZAMET (which permitted docetaxel use), demonstrate that docetaxel addition to enzalutamide + ADT only shows a survival benefit in the poorest prognosis group of men with synchronous, high volume disease; these findings are similar to those reported PEACE-1 and ARASENS. As such, it is important when developing a mHSPC treatment plan to consider the presentation and distribution of metastases, and the available options for ADT + a second/third agent: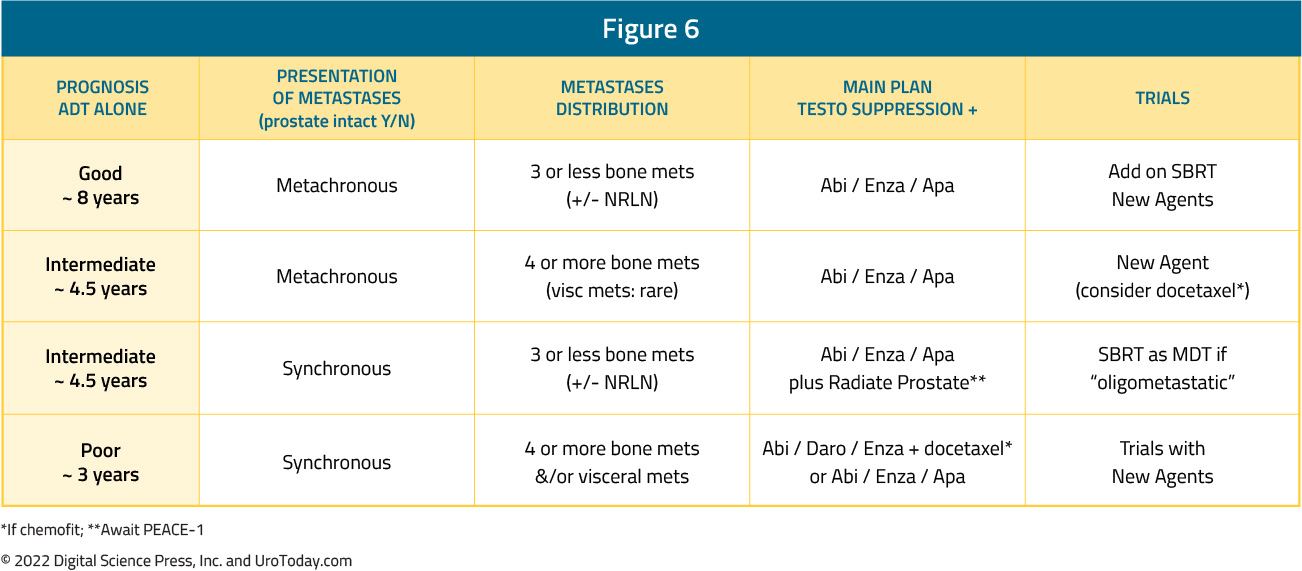
Published: August 2022


