(UroToday.com) The 2021 American Urologic Association (AUA) annual meeting included a guideline amendment update for non-muscle invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC) provided by Dr. James McKiernan. For each section of the guidelines (NMIBC and MIBC) Dr. McKiernan reviewed important guidelines highlights (that did not change in the amendment), as well as changes to the 2020 guidelines. The NMIBC guideline low, intermediate, and high-risk stratification initially proposed in 2016 was reviewed by the panel and deemed to be still accurate and relevant:

Variant histologies were also highlighted by Dr. McKiernan, notably Statement 6 – a genitourinary pathologist should review if any doubt with variant histology (ie. micropapillary, nested, plasmacytoid, neuroendocrine, sarcomatoid), extensive glandular differentiation, or the presence/absence of lymphovascular invasion (Moderate Recommendation; Evidence Strength: Grade C). Statement 8 – due to the high rate of upstaging with variant histology, clinicians should consider offering radical cystectomy (Expert Opinion).
With regards to BCG response, Dr. McKiernan highlighted several guideline statements that are unchanged from the previous guidelines. Statement 22 – in an intermediate- or high-risk patient with persistent or recurrence Ta or CIS disease after a single course of induction intravesical BCG, a clinician should offer a second course of BCG (Moderate Recommendation; Strength of evidence C). Guideline Statement 24 – in a patient fit for surgery with high-grade T1 disease after a single course of intravesical BCG, a clinician should offer radical cystectomy (Moderate Recommendation; Evidence Strength C).
Several statements from enhanced cystoscopy and surveillance were also noted by Dr. McKiernan. Statement 30 – a clinician should offer blue light cystoscopy at the time of TURBT, if available, to increase detection and decrease recurrence (Moderate Recommendation; Evidence Strength: Grade B). Statement 33 – for a low-risk patient whose first surveillance cystoscopy is negative, a clinician should perform subsequent surveillance cystoscopy six to nine months later and annually thereafter (Moderate Recommendation; Evidence Strength Grade C).
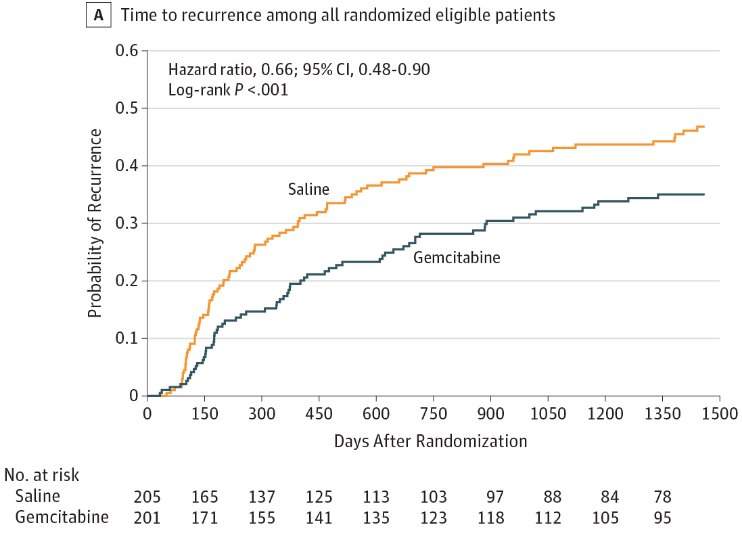
The NMIBC guideline update included a literature review and the amendment integrated into previous guidelines. The committee reviewed Ovid MEDLINE from July 1, 2015, through November 22, 2019, identifying 1,626 abstracts of which 76 met inclusion criteria. Statement 15 states – in low- or intermediate-risk bladder cancer, a clinician should consider administration of a single postoperative instillation of intravesical chemotherapy (ie. gemcitabine, mitomycin C) within 24 hours of TURBT. Previously, this was given a Moderate Recommendation; Evidence Strength: Grade B, however the update now gives this statement Evidence Strength: Grade A. This update is primarily based on the SWOG S0337 trial published by Messing et al.1 in 2018 whereby patients with suspected low-grade NMIBC were randomized to intravesical gemcitabine versus saline immediately following TURBT. Among 406 randomized eligible patients, 67 of 201 patients (4-year estimate, 35%) in the gemcitabine group and 91 of 205 patients (4-year estimate, 47%) in the saline group had cancer recurrence within 4.0 years (HR 0.66, 95% CI 0.48-0.90):
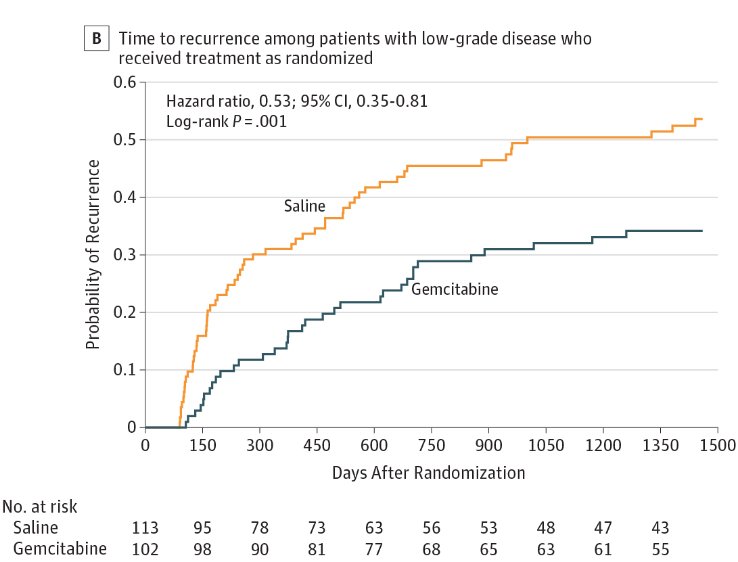
Among the 215 patients with low-grade NMIBC who underwent TURBT and drug instillation, 34 of 102 patients (4-year estimate, 34%) in the gemcitabine group and 59 of 113 patients (4-year estimate, 54%) in the saline group had cancer recurrence (HR 0.53, 95% CI 0.35-0.81):
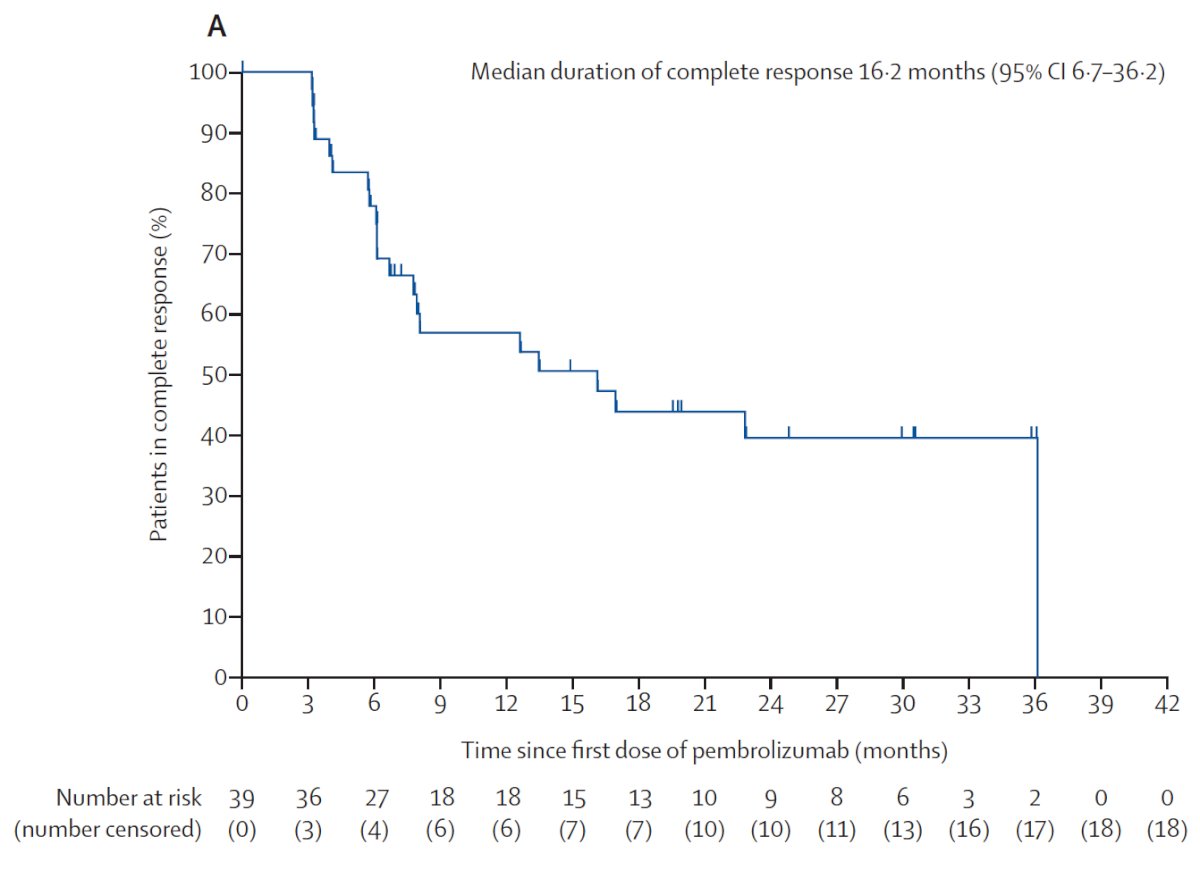
Statement 26 states – intermediate/high-risk NMIBC within 12 months of adequate BCG therapy unwilling or unfit for radical cystectomy, a clinician may recommend a clinical trial or offer alternative intravesical therapy (ie. valrubicin, gemcitabine, docetaxel, combination chemotherapy). A clinician may also offer systemic immunotherapy with pembrolizumab to a patient with CIS within 12 months of completion of adequate BCG therapy (Expert Opinion). Dr. McKiernan notes that when this amendment of the guideline was being completed, the KEYNOTE-057 study was just in abstract form, however, this study was recently peer-reviewed and published in Lancet Oncology.2 In KEYNOTE-057, 101 patients received pembrolizumab and 96 were included in the efficacy analysis (5 patients did not meet BCG-unresponsive criteria). The median age of patients was 73 years (range: 44-92), and patients received a median of 12.0 (range: 7.0-45.0) BCG instillations. Over a median follow-up of 36.4 months (IQR 32.0-40.7), 39 (41%; 95% CI 30.7-51.1) of 96 patients with BCG-unresponsive CIS of the bladder with or without papillary tumours had a complete response at 3 months:
For urinary biomarkers, Statement 9 states – in surveillance of NMIBC, a clinician should not use urinary biomarkers in place of cystoscopic evaluation (Strong Recommendation; Evidence Strength: Grade B). Dr. McKiernan notes that this statement has not changed, but emphasized that the panel acknowledges the uptake of Cxbladder in clinical practice. Although several studies have suggested that Cxbladder may replace cystoscopy there is currently a lack of high quality evidence to make this recommendation:

To conclude the NMIBC update, Dr. McKiernan discussed the current recommendations for “BCG unavailable” situations in order to maintain high-quality care in the absence of BCG:
- No BCG in low-risk NMIBC, intravesical chemo for intermediate-risk disease
- High-risk patients should have full strength induction BCG, however, if not available, then 1/2 to 1/3 dose is reasonable
- Limit BCG maintenance or forego maintenance if necessary
- Other options include gemcitabine, docetaxel, valrubicin, mitomycin, or gemcitabine/docetaxel
- Radical cystectomy should be undertaken for the highest risk (ie. high-grade T1, lymphovascular invasion, prostatic urethra involvement, variant histology)
Dr. McKiernan then discussed the MIBC portion of the AUA guideline update, starting with highlighting several statements that are important but have not changed. With regards to neoadjuvant therapy, statement 6 states – utilize a multidisciplinary approach and clinicians should offer cisplatin-based neoadjuvant chemotherapy to eligible radical cystectomy patients prior to cystectomy (Strong Recommendation; Evidence Level: Grade B). Statement 7 – clinicians should not prescribe carboplatin-based neoadjuvant chemotherapy. If ineligible for cisplatin-based neoadjuvant chemotherapy, patients should proceed to definitive locoregional therapy or a clinical trial (Expert Opinion).
Important statements for bladder preservation include Statement 22 – in patients undergoing consideration for bladder preserving therapy, maximal debulking TURBT and assessment of multifocal disease/CIS should be performed (Strong Recommendation; Evidence Level: Grade C). Statement 24 – clinicians should not offer radiation therapy alone as a curative treatment (Strong Recommendation; Evidence Level: Grade C).
Much like the NMIBC update, the MIBC update included a systematic review of the literature using Ovid MEDLINE from July 1, 2016, to May 18, 2020. There were 2,005 abstracts reviewed, of which 38 met inclusion criteria for consideration in the amendment. Statement 11 with regards to contiguous organs now states – when performing a standard radical cystectomy with curative intent, clinicians should remove the bladder, prostate, and seminal vesicles in males; clinicians should remove the bladder in females and should consider removal of adjacent reproductive organs based on the individual disease characteristics and need to obtain negative margins (Clinical Principle). Additionally, in select women with early-stage disease and desire to preserve fertility and/or sexual function, organ preservation may be considered as long as complete tumor resection can be achieved (Clinical Principle).
For neoadjuvant therapy, statement 8 now has a timeline advised for performing radical cystectomy after neoadjuvant chemotherapy, stating – clinicians should perform a radical cystectomy as soon as possible following a patient’s completion of and recovery from neoadjuvant chemotherapy (ideally within 12 weeks unless medically inadvisable) (Expert Opinion).
Dr. McKiernan concluded his presentation of updates from the NMIBC and MIBC guidelines with the following take-home messages:
- Review of these two critical guidelines reinforced that the majority of existing guideline recommendations were confirmed as accurate and relevant
- The committee identified several areas of research to improve care:
- Further defining the role of biomarkers
- Expansion of options for BCG unresponsive disease
- Integration of systemic immunotherapy in the treatment of MIBC
- Improved protocols for bladder preservation in MIBC
Presented by: James McKiernan, MD, John K. Lattimer Professor and Chair, Department of Urology, Columbia University/NY Presbyterian Hospital, New York, NY
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2021 American Urological Association, (AUA) Annual Meeting, Fri, Sep 10, 2021 – Mon, Sep 13, 2021.
References:
- Messing EM, Tangen CM, Lerner SP, et al. Effect of intravesical instillation of gemcitabine vs saline immediately following resection of suspected low-grade non-muscle-invasive bladder cancer on tumor recurrence: SWOG S0337 randomized clinical trial. JAMA. 2018 May 8;319(18):1880-1888.
- Balar AV, Kamat AM, Kulkarni GS, et al. Pembrolizumab monotherapy for the treatment of high-risk non-muscle-invasive bladder cancer unresponsive to BCG (KEYNOTE-057): An open-label, single-arm, multicenter, phase 2 study. Lancet Oncol. 2021 Jul;22(7):919-930.