(UroToday.com) High-risk non-muscle invasive bladder cancers (HR-NMIBC) that are unresectable by transurethral resection or refractory to BCG treatment are managed with radical cystectomy. The Keynote-057 study1 established the safety and efficacy of pembrolizumab in the BCG-refractory HR-NMIBC setting with 41% complete response rate. In this presentation, Dr. An presented results from the TRUCE-02 study (NCT04730232) testing the combination of chemotherapy (nab-paclitaxel) with the anti-PD-1 antibody tislelizumab in HR-NMIBC.
In this study, HR-NMIBC was defined as either a T1 tumor, high-grade Ta tumor, or carcinoma in situ. Multiple urologists were required to agree that the tumor was unresectable by TURBT.nThe schema of the presented study is shown below. Immunotherapy was administered on day 1 of the cycle and chemotherapy was administered on day 2. The primary endpoint of the study was complete response, with secondary endpoints of cystectomy-free survival, duration of response, and adverse events.
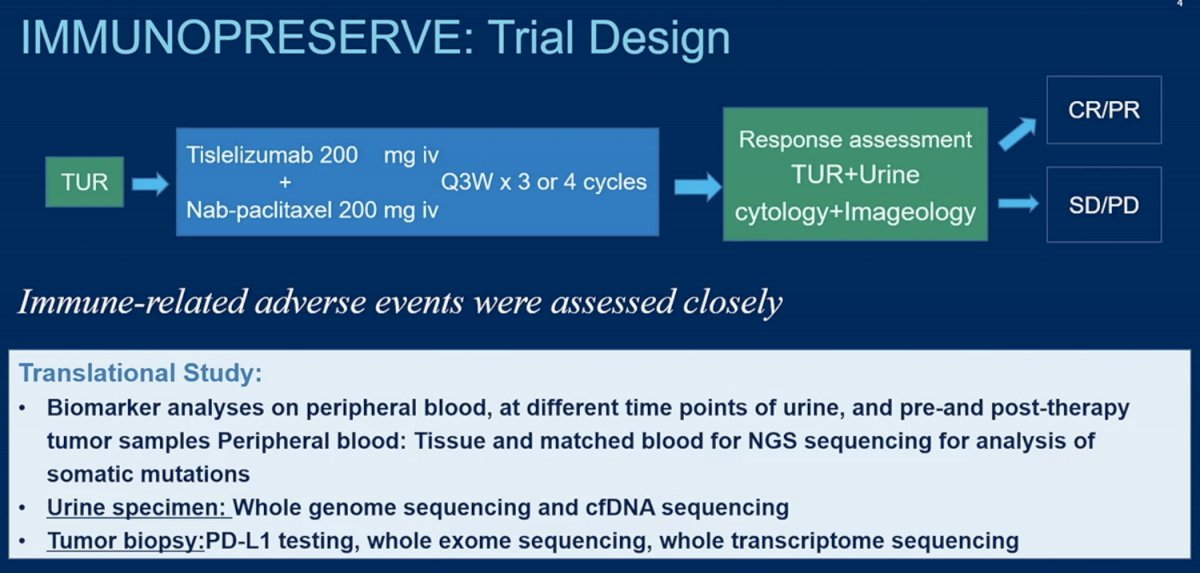
In total, 55 patients were enrolled, with 42 patients receiving at least three cycles (31 patients receiving 4 cycles) of both immunotherapy and chemotherapy and thus included in the final analysis. The median duration of follow-up was 9 months. Response data is shown below, the 55% of patients experiencing complete response and 40% of patients having either stable or progressive disease. A total of 28 patients have not required cystectomy, corresponding to a 62.7% 12-month bladder intact rate.
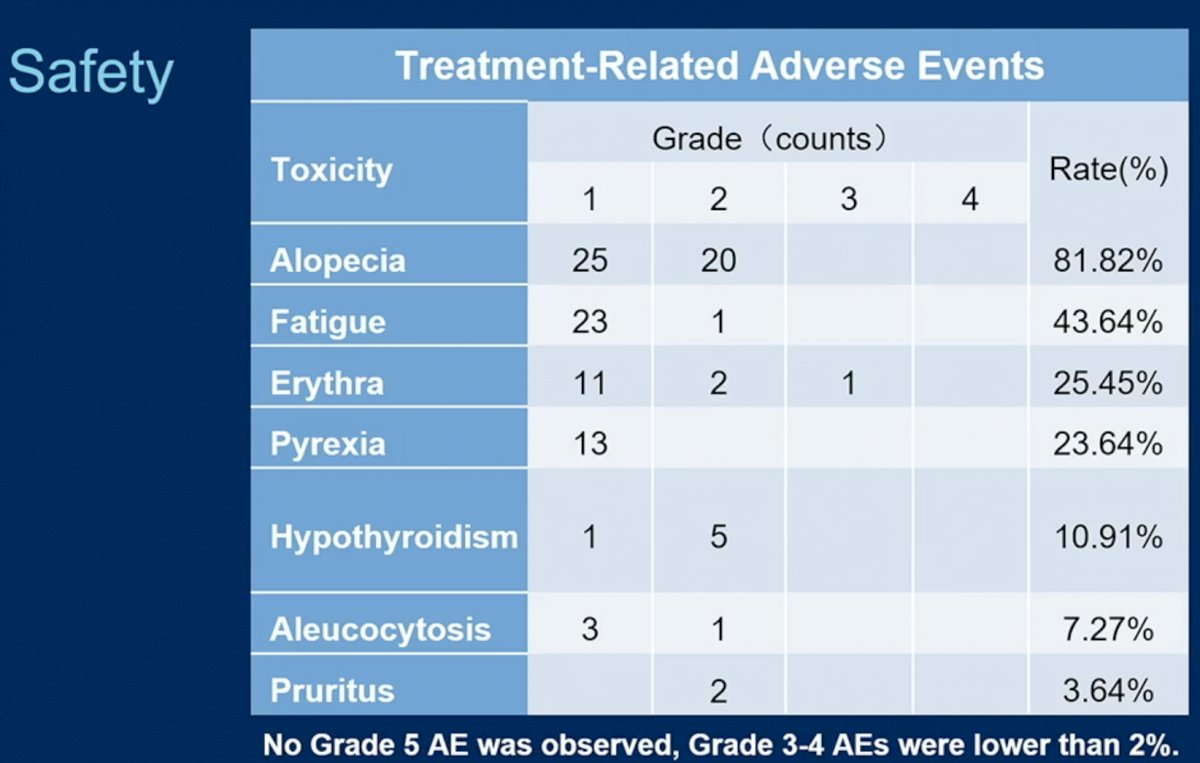
The major side effects from therapy are shown below.
With regards to biomarkers, there was no evidence correlation between PD-L1 tumor expression and response; 53% of overall responder tumors demonstrated PD-L1 expression, and 50% of non-responsive patients having tumors with PD-L1 expression. Within the responder group (CR or PR), 4/13 assayed patients had evidence of alteration in a homologous recombination repair gene, whereas no assayed patients in the non-responder group had such alterations. Furthermore, alterations in the AR and TCF7L2 DNA sequence were observed in over 40% of non-responders, but in no responders.


Dr. An concluded that the combination of tislelizumab and nab-paclitaxel may represent a feasible treatment option with satisfactory benefit and tolerable toxicity in HR-NMIBC. Biomarkers of response other than PD-L1 expression may be useful in determining which patients are most likely to benefit.
Presented by: Zesheng An, MD, PhD, MS, second hospital of Tianjin Medical University, Tianjin, China
Written by: Alok K. Tewari, MD, PhD, medical oncologist at the Dana-Farber Cancer Institute, @aloktewar on Twitter during the 2022 American Society of Clinical Oncology (ASCO) Annual Meeting, Chicago, IL, Fri, June 3 – Mon, June 7, 2022.
References:


