Executive Summary
Imaging is often used to evaluate men with biochemical recurrence (BCR) of prostate cancer after definitive primary treatment (radical prostatectomy [RP] or radiotherapy [RT]). The goal of imaging is to identify the source of elevated or rising serum prostate-specific antigen (PSA) levels because subsequent management depends on disease location and extent.
Salvage therapy (with surgery or radiation) may be considered for select cases with BCR to provide additional potential opportunity for cure. The salvage treatment strategy may be extended to regional adenopathy. Patients with limited distant metastases on imaging, referred to as oligometastatic disease (#5 demonstrable lesions), may be candidates for close observation, systemic hormonal therapy, or metastases-directed therapies with or without local therapy, depending on sites of recurrence. Patients with metastatic disease are typically treated with systemic therapy.
The purpose of this document is to describe the appropriate use of imaging in the diagnostic evaluation of patients with BCR after definitive primary treatment. The imaging modalities that were considered included CT, bone scan, and the U.S. Food and Drug Administration (FDA)–approved PET radiotracers that track malignancy-induced lipogenesis (11C-choline) and amino acid metabolism (18F-fluciclovine). The prostate-specific membrane antigen (PSMA)–targeted monoclonal antibody, 111In-capromab pendetide, is also included for historical perspective because it is neither available nor used clinically. The new class of PSMA-targeted PET radiotracers have generated considerable interest and are discussed briefly, although these agents are currently not approved for routine clinical use in the United States. Moreover, whole-body MRI (WB-MRI), with or without diffusion-weighted imaging, is excluded. Although WB-MRI may have utility in this clinical setting, particularly for the detection of bone metastases, the variability in availability, accessibility, quality, and standardization, as well as the fact that there are no currently established procedural terminology codes for reimbursement, has hindered its clinical adoption.1,2
Representatives from the Society of Nuclear Medicine and Molecular Imaging (SNMMI), the European Association of Nuclear Medicine (EANM), the American Society of Clinical Oncology (ASCO), the American College of Nuclear Medicine (ACNM), the American Society for Radiation Oncology (ASTRO), the American Urological Association (AUA), the American College of Physicians (ACP), the American College of Radiology (ACR), and the World Molecular Imaging Society (WMIS) assembled under the auspices of an autonomous workgroup to develop the following appropriate use criteria (AUC). This process was performed in accordance with the Protecting Access to Medicare Act of 2014.3 This legislation requires that all referring physicians consult AUC by using a clinical decision support mechanism before ordering advanced diagnostic imaging services. These services include diagnostic MRI, CT, and nuclear medicine procedures such as PET, among other services specified by the Secretary of Health and Human Services in consultation with physician specialty organizations and other stakeholders. The AUC herein are intended to aid referring medical practitioners in the appropriate use of imaging for the diagnostic evaluation of patients with BCR of prostate cancer after definitive primary treatment.
Prostate cancer is the second most commonly diagnosed cancer worldwide (13.5% of cancer diagnoses in men; 1,276,106 cases in 2018) and the fifth most common cause of cancer-related mortality among males (6.7%; 358,989 deaths in 2018).4 In the United States, prostate cancer is the most commonly diagnosed nonskin cancer in men (a projected 19% of all new cases of cancer; 164,690 cases in 2018) and the second most common cause of cancer-related mortality (a projected 29,430 deaths in 2018).5 Despite local definitive therapy, up to 40% of patients will develop recurrent disease.6 Most of these patients will have BCR with no evidence of metastasis on the basis of widely used standard imaging techniques (contrast-enhanced abdomen and pelvis CT, WB 99mTc-based bone scan, or pelvis multiparametric MRI), and the disease will manifest only with elevated serum PSA levels.
The definition of BCR (also referred to as PSA relapse) depends on the type of prior definitive therapy. In patients who have undergone RP, the AUA defines BCR when the serum PSA level is ≥ 0.2 ng/mL, measured 6–13 wk after surgery, and confirmed by a second determination of a PSA level of > 0.2 ng/mL.7 In patients treated with RT, the ASTRO Phoenix Criteria defines BCR as a rise in PSA level of 2 ng/mL or more above the nadir regardless of androgen deprivation therapy (ADT).8
The significance of biochemically recurrent disease varies considerably according to individual risk factors. One clinically important prognostic variable is PSA doubling time. For instance, prostate cancer–specific survival is approximately 90% in patients with a PSA doubling time of ≥ 15 mo (highest quartile), whereas it is about 20% for patients with a PSA doubling time of < 3 mo (lowest quartile).9 In part because of this wide variability in disease aggressiveness, coupled with competing causes of mortality and the typically long time to documented metastatic disease by standard imaging (median metastasis-free survival is 10 y in patients with BCR and no treatment), there is no defined standard management for this patient population.10 The development of metastasis in a patient signals that a change in treatment approach is warranted. Since the 1940s, the foundation of treatment for metastatic prostate cancer has been testosterone-lowering therapy. It is likely that the use of more sensitive imaging techniques will identify patients earlier who are at higher risk of developing overt metastases identified by more commonly used techniques. In some scenarios, earlier intervention in the disease process may result in improved outcomes for patients, as has been seen with postoperative RT.11
RT after a prostatectomy is commonly used to eradicate microscopic residual disease in the prostate bed, thereby reducing the risk of recurrence. Defining who needs postoperative RT is most often based on surgical pathology and postoperative PSA because standard imaging does not have sufficient sensitivity to identify early recurrences in the PSA range where salvage treatment is more likely to be curative. There is growing evidence that genomic biomarkers (e.g., Decipher, GenomeDx Biosciences, San Diego, CA) can have utility in this clinical setting, although it remains unclear as to how this information affects imaging choice.12,13 In the adjuvant setting, pathology (pT3a/b or surgical margins positive for disease) currently drives the addition of RT. In the salvage setting, when men have persistently detectable PSA (PSA persistence) or a delayed rise in PSA level (≥ 0.2 ng/mL), conventional imaging does not have sufficient sensitivity to identify early recurrences. The ability to detect residual or recurrent disease within the pelvis can affect RT dose and target. In the absence of molecular imaging, the question of whether to include pelvic lymph nodes in the RT field in patients with pathologic node-negative disease is a question that has been studied by the Radiation Therapy Oncology Group (RTOG) 0534 trial and is awaiting final results. The first report from RTOG 0534 (3-arm randomized trial) shows gains in freedom from progression with the addition of short-term (4–6 mo) ADT to prostate bed radiation and further gains with the inclusion of pelvic lymph node RT and short-term ADT over a PSA level of 0.34 ng/mL.14 With the ability to visualize prostate cancer cells, molecular imaging can help define RT treatment fields. Similarly, molecular imaging can identify patients who have early metastatic disease and could avoid RT to the prostate fossa. The use of molecular imaging to identify oligometastatic prostate cancer has allowed for additional treatment strategies in patient care.15 Studies show a benefit (e.g., biochemical progression-free survival, distant progression free survival) to metastasis-directed stereotactic body RT in the setting of oligometastatic prostate cancer.16-18 Molecular imaging can enhance the postoperative treatment algorithm for prostate cancer patients by identifying targets for RT.
This document is the product of an extensive literature search in combination with expert opinion. Its intent is to provide up-to-date information and recommendations for AUC for approved (in the United States) imaging technologies in the setting of BCR of prostate cancer after definitive treatment. We also discuss the outlook for upcoming imaging technologies that are anticipated to be approved in the United States relatively soon.
Methodology
Expert Workgroup Selection
The experts of this AUC workgroup were convened by the SNMMI to represent a multidisciplinary panel of health-care providers with substantive knowledge in the use of imaging evaluation of BCR of prostate cancer after definitive primary treatment. In addition to SNMMI members, representatives from ASCO, ASTRO, EANM, ACP, ACNM, AUA, ENETS, WMIS, and ACR were included in the workgroup. Fourteen physician members were ultimately selected to participate and contribute to the AUC. A complete list of workgroup participants and external reviewers can be found in Appendix A. Appendix B provides the disclosures and conflict of interest (COI) statements, and Appendix C describes the solicitation of public commentary.
AUC Development
The process for AUC development was modeled after the RAND/UCLA Appropriateness Method for AUC development.19 The process included the identification of a list of relevant clinical scenarios in which nuclear medicine can be used for imaging evaluation of BCR of prostate cancer after definitive primary treatment; a systematic review of evidence related to these clinical scenarios; and a systematic synthesis of available evidence, followed by the development of AUC for each of the various clinical scenarios by using a modified Delphi process. In addition, in this process we strove to adhere to the Institute of Medicine’s standards for developing trustworthy clinical guidance.20,21 The final document was drafted on the basis of group ratings and discussions.
Scope and Development of Clinical Scenarios
To begin this process, the workgroup discussed various potential clinical indications and applicable scenarios for the evaluation of BCR of prostate cancer after definitive primary therapy. For all indications, the relevant populations were patients with prostate cancer. The workgroup identified 2 clinical categories with 12 scenarios for this document. The categories are intended to be as representative of the relevant patient population as possible for the development of AUC. The resulting AUC are based on evidence and expert opinion regarding diagnostic accuracy and effects on clinical outcomes and clinical decision making as applied to each indication. Other factors affecting the AUC recommendations were potential harm—including long-term harm that may be difficult to capture—costs, availability, and patient preferences.
Systematic Review
ASCO conducted a systematic review to develop a comprehensive clinical practice guideline for optimum imaging strategies for advanced prostate cancer, and the same systematic review was used by the AUC workgroup. The workgroup selected the following key questions to guide the review:
1. What is the goal of imaging in advanced prostate cancer?
2. What imaging techniques are available for imaging advanced prostate cancer?
3. What are the unmet needs and potential impact of imaging according to different advanced prostate cancer disease states?
4. When and what type of imaging is appropriate in each scenario?
The inclusion and exclusion criteria for papers for this review were based on the study parameters established by the workgroup, using the PICOTS (population, intervention, comparisons, outcomes, timing, and setting) approach. A protocol for each systematic review defined parameters for a targeted literature search. Additional parameters included relevant study designs, literature sources, types of reports, and prespecified inclusion and exclusion criteria for the literature identified. The protocol for this guideline was reviewed and approved by the ASCO Clinical Practice Guidelines Committee’s Genitourinary Cancer Guideline Advisory Group.
PubMed and the Cochrane Collaboration Library electronic databases (with or without meeting abstracts) were searched for evidence that reported on outcomes of interest.
Data Extraction
Literature search results were reviewed and deemed appropriate for full text review by one ASCO staff reviewer in consultation with the expert panel cochairs (Edouard J Trabulsi, MD, Sidney Kimmel Medical College at Thomas Jefferson University, Philadelphia, PA, and Alberto Vargas, MD, Memorial Sloan Kettering Cancer Center, New York, NY). Data were extracted by 1 staff reviewer and subsequently checked for accuracy through an audit of the data by another ASCO staff member. Disagreements were resolved through discussion and consultation with the cochairs if necessary. Discrepancies were resolved through a consensus process.
Study Quality Assessment
Study quality was formally assessed for the studies identified. Design aspects related to the individual study quality were assessed by 1 reviewer and included factors such as blinding, allocation concealment, placebo control, intention to treat, funding sources, etc. The risk of bias was assessed as ‘‘low,’’ ‘‘intermediate,’’ or ‘‘high’’ for most of the identified evidence.
Database searches resulted in 6,378 potentially relevant abstracts. After dual review of abstracts and titles, 66 articles were selected for full-text dual review. Of these, 35 studies were determined to meet inclusion criteria and were included in this review, including 17 systematic reviews and 18 primary research papers.
Rating and Scoring
In developing these criteria, the workgroup members used the following definition of appropriateness to guide their considerations and group discussions: ‘‘The concept of appropriateness, as applied to health care, balances risk and benefit of a treatment, test, or procedure in the context of available resources for an individual patient with specific characteristics.’’ At the beginning of the process, workgroup members convened via webinars to develop the initial clinical indications. On evaluating the evidence summary of the systematic literature review, the workgroup further refined its draft clinical indications to ensure their accuracy and to facilitate consistent interpretation when scoring each indication for appropriateness. Using the evidence summary, workgroup members were first asked individually to assess the appropriateness and to provide a score for each of the identified indications. Workgroup members then convened in a group setting for several successive webinars to discuss each indication and associated scores from the first round of individual scoring. After deliberate discussion, a consensus score was determined and then assigned to the associated appropriate use indication. For this scoring round, the expert panel was encouraged to include their clinical expertise in addition to the available evidence in determining the final scores. All members contributed to the final discussion, and no one was forced into consensus. After the rating process was completed, the final appropriate use ratings were summarized in a format similar to that outlined by the RAND/UCLA Appropriateness Method.
The workgroup scored each indication as ‘‘appropriate,’’ ‘‘may be appropriate,’’ or ‘‘rarely appropriate’’ on a scale from 1 to 9. Scores 7–9 indicate that the use of the procedure is appropriate for the specific clinical indication and is generally considered acceptable. Scores 4–6 indicate that the use of the procedure may be appropriate for the specific indication. This implies that more research is needed to classify the indication definitively. Scores 1–3 indicate that the use of the procedure is rarely appropriate for the specific indication and is generally not considered acceptable.
As stated by other societies that develop AUC, the division of these scores into 3 general levels of appropriateness is partially arbitrary, and the numeric designations should be viewed as a continuum. In addition, if there was a difference in clinical opinion for an indication such that workgroup members could not agree on a common score, that indication was given a ‘‘may be appropriate’’ rating to indicate a lack of agreement on appropriateness on the basis of available literature and the members’ collective clinical opinion, indicating the need for additional research.
Clinical Categories and AUC Scores
Category 1. BCR after prior definitive treatment with RP or RT—initial imaging investigation
Category 2. BCR after prior definitive treatment with RP or RT—negative or equivocal results on initial standard imaging
Clinical Categories and AUC Scores
Table 1 presents the clinical category and final AUC scores for the use of imaging in the evaluation of BCR of prostate cancer after definitive primary treatment with RP or RT.
Table 1. Category 1: Clinical Scenarios for BCR After Prior Definitive Treatment with RP or RT—Initial Imaging Investigation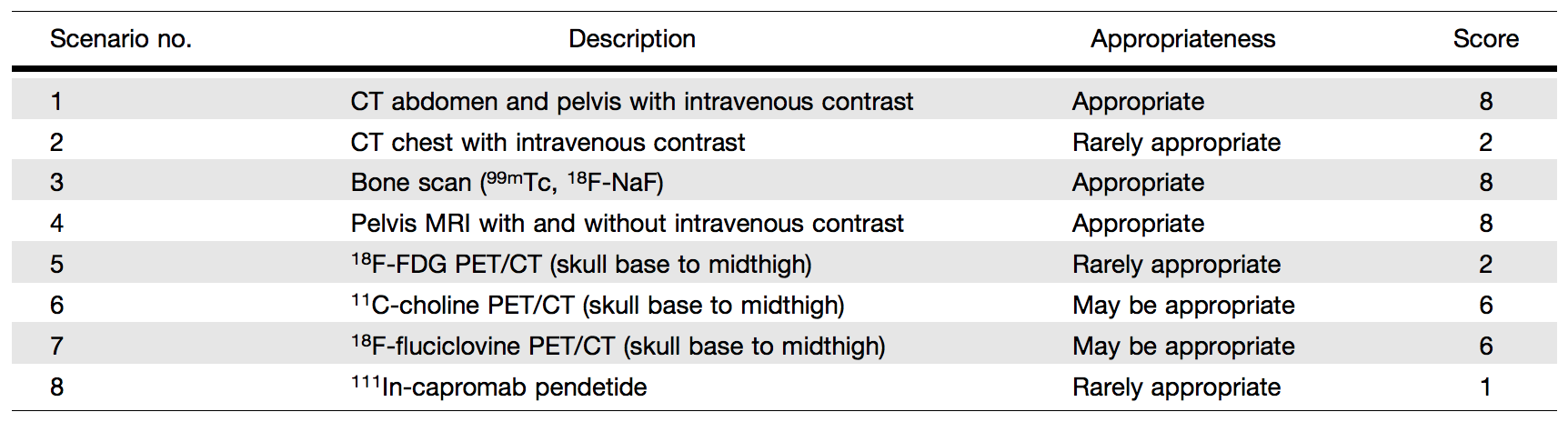
Table 2 presents the clinical category and final AUC scores for the use of imaging in the evaluation of BCR of prostate cancer after definitive primary treatment with RP or RT, with negative or equivocal results on standard imaging.
Table 2. Category 2: Clinical Scenarios for BCR After Prior Definitive Treatment with RP or RT—Negative Or Equivocal Results on Initial Standard Imaging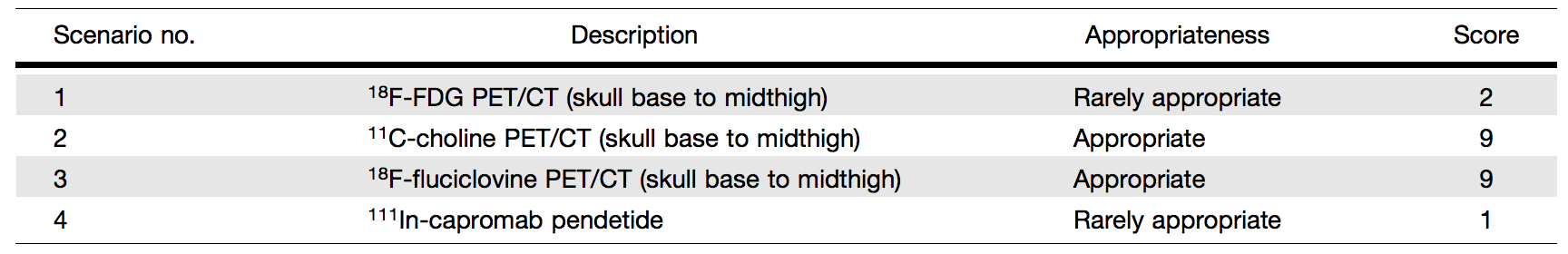
Category 1, Scenario 1: CT of the Abdomen and Pelvis with Intravenous Contrast (Score 8 – Appropriate). An abdominal and pelvis CT in prostate cancer treatment follow-up is used to focus on the assessment of metastatic disease in the lymph nodes, bone, and visceral organs. In the evaluation of nodal disease, CT relies on nodal size to detect tumors. Using a short-axis diameter of 1.0 cm as a cut point, studies have reported sensitivities of between 27% and 75% with specificities of between 66% and 100%.22 However, the sensitivity of abdominopelvic CT for the detection of low-volume recurrent disease is limited, particularly when PSA levels are low. Studies have shown CT results to be positive in only 11%–14% of men with biochemical relapse after RP.23 The mean PSA value associated with positive results for disease in a CT examination was 12.4 ng/mL, and the mean PSA velocity was 30.6 ng/mL/y.24 The usual pattern of vertical nodal spread beginning in the pelvis can be absent in nearly 75% of patients with disease recurrence after treatment.25 In these patients, most of whom have undergone previous pelvic lymph node dissection at the time of RP, only retroperitoneal adenopathy is commonly detected by CT. In addition, CT is useful to detect advanced disease in bone and visceral metastases and in RT treatment planning to define the prostate bed and locoregional and distant metastatic target volumes. Bone lesions from prostate cancer are often seen as sclerotic lesions, although there are numerous other benign causes for dense bone lesions. A bone scan is superior to CT in the diagnosis and follow-up of bone metastases, as it provides functional information about a bone lesion. In summary, despite the recognized limitations of an abdominopelvic CT, it is readily available at relatively low cost and has traditionally been considered as standard imaging in this clinical setting, which prompted the panel to recommend an appropriateness score of 8 (appropriate).
Category 1, Scenario 2: CT of the Chest With Intravenous Contrast (Score 2 – Rarely Appropriate). Lung metastasis from prostate cancer is relatively uncommon. In an autopsy series, the relative ratio frequency of lung involvement was 14.2%–19.8%.26 Moreover, most lung metastases appear later in the disease and not early in the recurrence setting. Therefore, the panel recommended that CT of the chest receive an appropriateness score of 2 (rarely appropriate).
Category 1, Scenario 3: Bone scan (99mTc-Methylene Diphosphonate [MDP] WB Scan, 18F-Sodium Fluoride [NaF] PET/CT) (Score 8 – Appropriate). In the clinical setting of primary staging, current National Comprehensive Cancer Network (NCCN) guidelines recommend an imaging evaluation with a bone scan in any patient with a PSA level of > 20 ng/mL, a Gleason score of 8 or greater, or a clinical stage of T3 or greater (high-risk and very highrisk groups) and in patients with any of 2 of the following: a PSA level of > 10 ng/mL, a Gleason score of 7 or over, and a clinical stage of T2b/T2c or greater (intermediate-unfavorable group). A recent systematic review of 54 studies encompassing a total sample size of 20,421 patients with treatment-na¨ıve cancer found yield rates of 4% with a PSA level of ≤ 10 ng/mL, 7% with a PSA level of 10 to ≤ 20 ng/mL, 42% with a PSA level of > 20 ng/mL, 4.1% with a Gleason score of 6 or less, 10% with a Gleason score of 7, and 28.79% with a Gleason score of 8 or greater.27 In subgroup analyses, a Gleason score of 7 with a PSA level of < 20 ng/mL had a 3% yield, whereas a Gleason score of 8 with a PSA level of ≤ 10 ng/mL had a yield of 20%, suggesting that a bone scan would be useful with a PSA level of > 20 ng/mL or a Gleason score of 8 or over.
However, it is probable that the case for patients with BCR of prostate cancer is different. One study of 1,197 patients who had undergone RP found that those with a positive bone scan result always had a PSA level of at least 7 ng/mL,28 and another study of 100 patients after RP suggested an optimal trigger PSA level cutoff of 30–40 ng/mL.29 One study of 142 patients with PSA levels of up to 1 ng/mL after RP reported only a 2% bone scan yield.30 Therefore, these investigations suggest that the PSA trigger cutoff for a positive bone scan result in patients who have undergone RP may be in the range of 7–30 ng/mL and not lower.
The PSA velocity (i.e., the rate of change of serum PSA levels over time) may also be relevant. A study of 132 patients after RP suggested that the PSA velocity was more important, with 0.5 ng/ mL/mo serving as an optimal cutoff.23 A study of 292 patients, most of whom had undergone RP, suggested a trigger PSA value of 5 ng/mL and a PSA doubling time of 10 mo,31 whereas another study of 128 patients after RP suggested cutoffs of 10 ng/mL for the PSA value and 6 mo for the PSA doubling time.32 Another investigation of 438 patients after RP also incorporated the presence or absence of ADT. Whereas with patients before being treated with ADT, a threshold PSA doubling time of 9 mo was a fairly effective cutoff (yield of 1%–5% for > 9 mo vs. 11%–44% for < 9 mo), for patients after ADT treatment, there was a yield of at least 10%, even with long PSA doubling times and low PSA levels (below 10 ng/mL).33 A study of 239 patients used trigger PSA values and PSA slope and velocity to create a monogram.34 The results concurred with the NCCN guidelines in recommending a bone scan with a PSA level of 20 ng/mL, or with a PSA level of 10 ng/mL with a Gleason score of 7 or greater or stage T2 or greater; a PSA doubling time of 9 mo or less was added as another indication.
For 18F-NaF PET, dedicated studies that focus specifically on recurrence are few, and these studies do not separate patients who have undergone RP from those who have undergone RT. Theoretically, the higher photon flux and coincidence detection with PET and concurrent CT should increase sensitivity and specificity, respectively, over a planar WB scan, and multiple studies, albeit mostly for initial staging35 or mixed indications of initial staging and BCR.36-38 Interestingly, 1 study showed a decline in specificity from 82% to 54%,39 whereas another showed a small decrease from 88% to 82% vis-à-vis SPECT, but with an overall improvement in both sensitivity and specificity over a planar bone scan.40 Moreover, a large retrospective study of the National Oncologic PET Registry found a change in management over a bone scan in 12%–16% of cases.41 A recent study of 62 patients with mixed indications suggests a PSA cutoff of 6 ng/mL for previously treated patients, lower than that previously suggested for a bone scan.42
A few studies have compared 18F-NaF to other PET tracers. These generally do not separate RP from RT.43 Results of studies that compared 18F-NaF to 18F-fluorocholine are mixed. Some show increased sensitivity (for bone lesions) at the expense of specificity.39,43 One study that focused on initial staging found a similar performance for bone lesions,44 whereas another, with a mix of initial and recurrent indications, showed some loss in specificity with 18F-NaF.45 When 18F-FDG and 18F-NaF are compared, the latter is more sensitive for detecting bone metastases at BCR even at PSA levels as low as 2–4 ng/mL, albeit at the expense of specificity.46-48 For PSMA tracers, mostly in a mixed primary and recurrent population, studies show a similar pattern, with 18F-NaF detecting more bone lesions at the expense of decreased specificity;49-51 1 study showed no significant difference.52 A consistent result is that, compared with other PET tracers, 18F-NaF is more sensitive for bone lesions at the expense of specificity. It outperforms conventional 99mTc-based bone scans, which may be relevant in clinical management decisions.53 In summary, a bone scan is considered standard imaging and received an appropriateness score of 8 (appropriate).
Category 1, Scenario 4: MRI of the Pelvis With And Without Intravenous Contrast (Score 8 – Appropriate). An MRI of the pelvis can be effective in identifying sites of recurrent prostate cancer and its use is rapidly increasing (54). Most studies demonstrate that an MRI of the pelvis is reliable for the detection of local recurrence either at the site of the prostate bed in patients who have undergone RP or within the prostate in patients after RT treatment.55-59 The combination of diffusion-weighted, T2- weighted, and dynamic contrast enhancement MRI is particularly effective for detecting local recurrence.58,60 For pelvic nodal metastatic detection, a pelvic MRI has similar limitations to that of CT, namely, low sensitivity due to the dependence on size criteria. Many lymph nodes that test positive are too small to meet the 0.8- to 1-cm size threshold for positivity on MRI. Although there was initial enthusiasm for diffusion-weighted imaging for detecting normal-sized lymph nodes at initial staging, there is no evidence in the literature that this method is valid in patients with BCR,61 and the method has proven difficult outside of research settings. When lesions are present in the pelvic bones, MRI is highly sensitive, equaling PET scans in this regard, with the caveat that findings may not be specific for bone metastases.58 MRI can be predictive of response to salvage RT from the extent of the recurrent disease.62 Thus, a pelvic MRI provides useful information, in particular for local recurrence and bone metastases in the setting of BCR, which led to an appropriateness score of 8 (appropriate).
Category 1, Scenario 5: 18F-FDG PET/CT (Skull Base to Midthigh) (Score 2 – Rarely Appropriate). Category 2, Scenario 1: 18F-FDG PET/CT (Skull Base to Midthigh) (Score 2 – Rarely Appropriate). 18F-FDG PET/CT has revolutionized the field of cancer imaging and has become one of the pillars of management of many cancers. This huge success is not reflected in prostate cancer, where many studies have documented disappointing detection capabilities or better alternative imaging tests. This is despite some results in the literature suggesting the potential utility of 18F-FDG PET/CT in prostate cancer; this discrepancy is likely because of variability in the standards of reference used or changing paradigms in the management of BCR. For example, using standard definitions, Öztürk and Karapolat63 evaluated 18FFDG PET/CT in 28 patients with BCR after RP or RT and found that imaging results were negative in 16 (57.1%) patients and positive in 12 (42.9%). However, no summary PSA statistics for the study group were included, and no mention of biopsy confirmation was made or other measures provided to assess the true positivity of the PET findings. Schöder et al.64 reported sensitivities of 71%–80% and specificities of 73%–77% for 18F-FDG PET in the recurrence setting, where the median PSA level was 2.4 ng/mL. These results probably overestimate the clinical utility of 18F-FDG PET/CT, given that many of the patients had positive findings on other standard imaging and the PSA thresholds are considerably above those that would trigger salvage RT in the contemporary setting (typically around 0.5 ng/mL). In a subset of patients with early BCR after RP (PSA level < 1 ng/mL), a more recent study reported 18F-FDG PET positivity in only 1 of 5 patients; on directed biopsy, only inflammatory tissue was identified at the site of 18F-FDG uptake in the thoracic spine (i.e., false positive).30 Jadvar et al.46 found 18F-FDG PET/CT detection rates of only 8.1% in a prospective study of 37 patients with BCR and negative results of standard imaging. The same group published a comparative performance study of PET tracers in prostate cancer BCR and found that 18F-FDG PET/CT exhibited the lowest detection rates compared with those of 11C-acetate, 11C- or 18Fcholine, anti-1-amino-3-18F-fluorocyclobutane-1-carboxylic acid (FACBC or 18F-fluciclovine), and radiolabeled ligand targeted to PSMA.65 However, 18F-FDG PET/CT may play a role later in the course of prostate cancer, particularly in the context of metastatic disease.66-69 In summary, 18F-FDG PET/CT is rarely appropriate for the evaluation of BCR of prostate cancer after RP or RT, even in the context of negative or equivocal standard imaging results, leading to an appropriateness score of 2 (rarely appropriate).
Category 1, Scenario 6: 11C-Choline PET/CT (Skull Base to Midthigh) (Score 6 – May be Appropriate). Category 2, Scenario 2: 11C-Choline PET/CT (Skull Base to Midthigh) (Score 9 – Appropriate). 11C-choline PET/CT has long been used in BCR and is currently incorporated into NCCN and European Association of Urology guidelines. 11C-choline was approved in the United States on September 12, 2012, for PET imaging in recurrent prostate cancer.70 The fluorinated choline radiotracer (18F-fluorocholine) has also been investigated relatively extensively and is used clinically in many countries; however, the radiotracer is not FDA approved. Although the literature on 11C-choline PET/CT is relatively robust, most reports are retrospective and rarely compare 11C-choline PET/CT to standard imaging (abdominopelvic CT, bone scan, and pelvis MRI). This is particularly true for patients with prior definitive treatment with RT, for which only 2 retrospective studies have been reported.71,72 A first meta analysis provided a pooled sensitivity of 85.6% (95% confidence interval [CI]: 82.9%–88.1%) and a pooled specificity of 92.6% (95% CI: 90.1%–94.6%) for all sites of disease.73 A more recent meta analysis,74 which considered only 11C-choline, reported a pooled sensitivity of 89% (95% CI: 83%–93%) and a pooled specificity of 89% (95% CI: 73%–96%). For local relapse, the pooled sensitivity was 61% (95% CI: 40%–80%) and the pooled specificity 97% (95% CI: 87%–99%); for nodal disease, the pooled detection rate was 36% (95% CI: 22%–50%), whereas for bone metastases, the pooled detection rate was 25% (95% CI: 16%–34%). As with all PET imaging methods, choline PET/CT sensitivity is strongly dependent on the PSA level and kinetics (velocity, doubling time, acceleration).75 In patients with BCR after RP, choline PET/CT detection rates are only 5%– 24% when the PSA level is < 1 ng/mL, but rises to 67%–100% when the PSA level is > 5 ng/mL. Therefore, a PSA cutoff level of between 1 and 2 ng/mL has been suggested for choline PET/CT imaging. It may also be advantageous to consider PSA kinetics rather than PSA levels.76 In balancing its strengths (relatively abundant literature despite its stated limitations, FDA approval, incorporation into patient management guidelines) and weaknesses (need for on-site cyclotron and hence less accessibility, relatively high cost), the panel assessed that 11C-choline PET/CT may be appropriate (appropriateness score of 6) for the first imaging approach in patients with BCR in comparison to the widely available and less costly standard imaging. However, in patients with negative or equivocal conventional imaging results, the appropriateness score was raised to 9 (appropriate).
Category 1, Scenario 7: 18F-Fluciclovine PET/CT (Skull Base to Midthigh) (Score 6 – May be Appropriate). Category 2, Scenario 3: 18F-Fluciclovine PET/CT (Skull Base to Midthigh) (Score 9 – Appropriate). 18F-fluciclovine (Axumin) was FDA approved on May 26, 2016, for PET imaging in men with suspected prostate cancer recurrence based on elevated PSA levels after prior treatment.77 It was prospectively shown in 89 patients that 18Ffluciclovine, in comparison to 11C-choline, is generally superior for detection of recurrence, especially for PSA values of , 2 ng/mL (18F-fluciclovine vs. 11C-choline: 21% vs. 14% for PSA level of , 1 ng/mL, 29% vs. 29% for PSA level of 1 to , 2 ng/mL, 45% vs. 36% for PSA level of 2 to , 3 ng/mL, and 59% vs. 50% for PSA level of $ 3 ng/mL).78 The overall sensitivity, specificity, and positive predictive values were 37%, 67%, and 97%, respectively, for 18F-fluciclovine and 32%, 40%, and 90%, respectively, for 11C-choline. In a large multisite study with 596 patients, an overall detection rate of 68% was reported. 18F-fluciclovine uptake suspicious for disease recurrence was found in the prostate bed and pelvic lymph node regions in 39% and 33% of scans, respectively. Metastatic involvement outside the pelvis was detected in 27% of scans. The corresponding positive predictive value was 62% for all detected lesions, with 92% for extraprostatic involvement and 72% for prostate/bed involvement.79 Another recent study that focused on patients with a PSA level of # 1 ng/mL reported an overall positive lesion detection rate of 46.4%, with local and nodal recurrences detected more often than distant metastases, and with a Gleason score of greater than 7 associated with positive scan results.80 The use of 18F-fluciclovine PET/ CT has an impact on the clinical management of patients with BCR of prostate cancer. The prospective multicenter LOCATE trial reported a change in management in 59% of patients. Within this cohort, there were changes from salvage or noncurative systematic therapy to watchful waiting in 25% of patients, from noncurative systematic therapy to salvage therapy in 24%, and from salvage therapy to noncurative systemic therapy in 9%.81 Another investigation reported change in salvage RT management of 41% of patients who had undergone a prostatectomy.82 Although not as sensitive as PSMA-targeted PET agents, 18F-fluciclovine is nevertheless approved in the United States in the setting of recurrent disease. Similar to its consideration of 11C-choline, the panel assessed that 18F-fluciclovine PET/CT may be appropriate (appropriateness score of 6) as the first imaging approach in patients with BCR in comparison to the widely available and lower cost standard imaging. However, in the setting of negative or equivocal conventional imaging results, the panel recommended a score of 9 (appropriate) for 18F-fluciclovine.
Category 1, Scenario 8: 111In-Capromab Pendetide (Score 1 – Rarely Appropriate). Category 2, Scenario 4: 111In-Capromab Pendetide (Score 1 – Rarely Appropriate). 111In-capromab pendetide is a radioimmunoconjugate consisting of the murine IgG1 k-monoclonal antibody capromab (7E11-C5.3) conjugated to the linker-chelator glycyl-tyrosyl-(N,-diethylenetriaminepentaacetic acid)- lysine hydrochloride (GYK-DTPA-HCl) and labeled with radioisotope 111In, with ligand-binding and g-emitting activities. It binds to a cytoplasmic epitope of human PSMA, a cell transmembrane glycoprotein abundantly expressed by prostate epithelium, and is typically overexpressed by prostate cancer cells.83 Radioimmunoscintigraphy imaging with 111In-capromab pendetide was approved by the FDA on October 28, 1996, as a diagnostic imaging agent in newly diagnosed patients with biopsy-proven prostate cancer.84
The utility of imaging with 111In-capromab pendetide for prostate cancer has been the subject of continual debate since its approval. Its disappointing low levels of both sensitivity and specificity significantly limited its use and acceptance. This seems to be an inherent property of the labeled antibody, which has not been shown to yield progressively better accuracy with the experience of the image interpreter, likely because of the agent’s dependence on cytoplasmic binding, which achieves better results with nonviable than with viable tumor tissue. Another major limitation of this agent is that the antibody remains in the blood, leading to high background signals and consequently reduced target-to-background ratios and detection rates.
In a study of 30 men with biochemical relapse after prostatectomy who received salvage RT, 111In-capromab pendetide scan results were compared with postsalvage RT PSA response.85 In these patients, presalvage RT 111In-capromab pendetide scan findings outside the prostate fossa were not predictive of biochemical control after RT. Pucar et al.86 concluded that 111In-capromab pendetide had ‘‘no added benefit over other imaging modalities [available at that time] in evaluating postradical prostatectomy recurrence, due to its low sensitivity for detecting local recurrences and bone metastases.’’ Another study evaluated 111In-capromab pendetide against 18F-fluciclovine.87,88 It found that PET/CT with 18F-fluciclovine demonstrated superior sensitivity, specificity, and accuracy to that of 111In-capromab for the detection of disease, both in the prostatic bed and in extraprostatic sites.
Notably, despite FDA approval and widespread use of 111Incapromab pendetide in the United States for more than 22 y, many health insurance providers will still not provide standard insurance coverage for imaging with 111In-capromab pendetide for prostate cancer, which continues to be categorized as ‘‘investigational,’’ with the notation that the current medical literature ‘‘is insufficient to support conclusions concerning efficacy, optimal use and impact on the diagnosis, treatment or clinical management of prostate cancer using radioimmunoscintigraphy imaging with 111In capromab pendetide’’.89,90 Thus, 111In-capromab pendetide (marketed exclusively as) is no longer recommended in the setting of BCR. As of July 9, 2018, the FDA also reports on their website that Aytu BioScience, the manufacturer of ProstaScint, reported voluntary discontinuation of the product.91 As a result, the panel assigned an appropriateness score of 1 (rarely appropriate) to 111In-capromab pendetide.
Qualifying Statements
Special Commentary
In addition to the currently approved radiotracers for imaging of prostate cancer (18F-fluciclovine and 11C-choline), a new class of radiotracers has been developed that targets the PSMA.92,93 The most commonly used compound is 68Ga-PSMA-11, which is limited in production and distribution, as it is labeled with 68Ga (half-life 5 68 min) and is not yet approved in the United States.94,95 68Ga-PSMA-11 has been shown to have a higher detection sensitivity compared with that of 18F-fluorocholine96,97 and has also recently been compared with 18F-fluciclovine and shown to be superior in lesion detection.98,99 Recently, a 635-patient single-arm clinical trial of 68Ga-PSMA-11 demonstrated substantial interreader reproducibility and high detection sensitivity and accuracy compared with a composite endpoint in patients with BCR.100 68Ga-PSMA-11 PET localized recurrent prostate cancer in 75% of patients; detection rates significantly increased with PSA level: 38% for < 0.5 ng/mL, 57% for 0.5 to < 1.0 ng/mL, 84% for 1.0 to < 2.0 ng/mL, 86% for 2.0 to < 5.0 ng/mL, and 97% for ≥ 5.0 ng/mL. PSMA PET resulted in changes in RT plans in 53% of patients undergoing definitive RT.101,102 In the salvage setting, Calais et al.103,104 showed that of 270 patients with a PSA level of < 1 ng/mL, use of 68Ga-PSMA11 PET/CT had a major impact on RT planning in 19%, justifying a randomized imaging trial of salvage RT.
Although much of the data with PSMA-targeted PET radiotracers have focused on 68Ga-labeled agents, the use of 18F as a radionuclide has several advantages, including nearly unlimited cyclotron-based production, feasible central distribution due to a 110-min physical half-life (vs. 68 min for 68Ga), higher positron yield, and lower positron energy (leading to shorter positron annihilation distances and higher spatial resolution).105,106 These intrinsic advantages may lead to the widespread adoption of 18F-labeled ligands as the worldwide demand for PSMA-targeted radiotracers continues to increase. 18F-labeled PSMA-targeted radiotracers have shown high sensitivity for the detection of putative sites of prostate cancer in men with BCR after attempted curative therapy. More recently, Giesel et al.107 used a different 18F-labeled radiotracer known as 18F-PSMA-1007 in a retrospective analysis of 251 patients with BCR of prostate cancer. This tracer exhibits more hepatic and less renal excretion, potentially simplifying evaluation of the pelvis. In total, 204 of 251 (81.3%) patients had findings on 18F-PSMA-1007 PET deemed to be evidence of a site or sites of recurrent disease. The patient detection efficiency at the PSA range of 0.2–0.5 ng/mL was 40 of 65 (61.5%). In another prospective investigation with 18F-DCPyL PET/CT in 31 patients with BCR after RP, the positive detection rate was 59.1% in patients with a PSA level of < 1.0 ng/mL and 88.9% in patients with a PSA level of > 1.0 ng/mL.108 Rousseau et al.109 reported a similar high detection efficacy with 18F-DCFPyL in 130 patients with BCR after curative intent primary therapy, with positive findings in 60% (PSA level ≥ 0.4 to < 0.5 ng/mL), 78% (≥ 0.5 to < 1.0 ng/mL), 72% (≥ 1.0 to < 2.0 ng/mL), and 92% (≥ 2.0 ng/mL) of cases. Currently, it is unclear whether there is a benefit of one PSMA targeted agent over another, but because of the physical advantages of 18F-labeled compounds, they will likely play a dominant role after they have been approved and become available.
In summary, PSMA PET is anticipated to have a significant role in the imaging evaluation of patients with BCR given its higher sensitivity and accuracy, although currently we are awaiting approval of these agents in the United States. Aside from regulatory approval, ongoing and future prospective investigations will be needed to examine how PSMA-based theranostics provide added clinical value and have an impact on treatment strategy, patient outcome, and relative economic outlay.110
Implementation of the AUC Guidance
SNMMI has been developing the AUC for high-value nuclear medicine procedures since early 2015. This initiative was primarily undertaken to assist referring physicians and ordering professionals fulfill the requirements of the 2014 Protecting Access to Medicare Act (PAMA). Section 218(b) of PAMA established a new program under the statute for fee-for-service Medicare to promote the use of AUC for Advanced Diagnostic Imaging Services (ADIS), including CT, MRI, and all nuclear medicine procedures such as PET. PAMA requires referring physicians to consult AUC developed by a Centers for Medicare and Medicaid Services (CMS)–approved qualified provider-led entity, or Q-PLE, to ensure cost-effective and appropriate use of ADIS. After going through a rigorous and extensive application that required SNMMI to document their guideline development process, including COI adjudication and composition of expert panels, the society was approved as a Q-PLE in June 2016.
The PAMA legislation requiring the development of AUC also stipulated the mechanism of their delivery through a ‘‘qualified clinical decision support mechanism’’ (Q-CDSM) before ordering any advanced imaging. Therefore, successful implementation and complete adoption of this program relies on integration of AUC developed by PLEs into these Q-CDSMs. The society has partnered with leading CDSM providers to facilitate the adoption and use of SNMMI AUC.
Final implementation of the AUC program has been delayed until January 2020, in part so that the CMS can issue more substantive guidance for the priority clinical areas and exceptions for the ordering professionals for whom consultation with AUC would pose significant hardship. Delaying implementation also provides more preparation time for the referring physicians and health-care institutions to comply with the legislative requirements.
Acknowledgments
The workgroup acknowledges staff support from the Pacific Northwest Evidence-Based Practice Center of Oregon Health and Science University (Roger Chou, MD, FACP, Director, and Miranda Pappas, MA, Project Manager, Research Associate).
Appendix A: Workgroup Members and External Reviewers
Workgroup
The members of the workgroup are Hossein Jadvar, MD, PhD, MPH, MBA (Chair), University of Southern California Keck School of Medicine, Los Angeles, CA (SNMMI); Leslie K. Ballas, MD, University of Southern California Keck School of Medicine, Los Angeles, CA (ASTRO); Peter L. Choyke, MD, National Institutes of Health, Bethesda, MD (ASCO); Stefano Fanti, MD, University of Bologna, Bologna, Italy (EANM); James L. Gulley, MD, PhD, National Institutes of Health, Bethesda, MD (ACP); Ken Herrmann, MD, Department of Nuclear Medicine, Universit¨atsklinikum Essen, Essen, Germany, and Department of Molecular and Medical Pharmacology, David Geffen School of Medicine at UCLA, Los Angeles, CA (EANM); Thomas A. Hope, MD, University of California San Francisco, San Francisco, CA (SNMMI); Alan K. Klitzke, MD, Roswell Comprehensive Cancer Center, Buffalo, NY (ACNM); Jorge Oldan, MD, University of North Carolina, Chapel Hill Hospitals, Chapel Hill, NC (ASCO, SNMMI); Martin G. Pomper, MD, PhD, Johns Hopkins University Medical School, Baltimore, MD (WMIS); Steven P. Rowe, MD, PhD, Johns Hopkins University Medical School, Baltimore, MD (SNMMI); Rathan M. Subramaniam, MD, PhD, MPH, University of Texas Southwestern Medical Center, Dallas, TX (ACNM, ACR); Samir S. Taneja, MD, NYU Langone Medical Center, New York, NY (AUA); Herbert Alberto Vargas, MD, Memorial Sloan Kettering Cancer Center, New York, NY (ASCO).
External Reviewers
The external (peer) reviewers were Soroush Rais-Bahrami, MD, University of Alabama, Birmingham, AL; Andrei Purysko, MD, Cleveland Clinic, Cleveland, OH; Jefferey Weinreb, MD, Yale New Haven Hospital, New Haven, CT; Laura Evangelista, MD, PhD, Istituto Oncologico Veneto IOV – IRCCS Padova, Italy; Bridget F. Koontz, MD, Duke University, Durham, NC; Mack Roach III, MD, University of California, San Francisco, CA.
SNMMI
The supporting staff from SNMMI are Sukhjeet Ahuja, MD, MPH, Sr. Director, Health Policy & Quality Department; Teresa Ellmer, MIS, CNMT, Senior Program Manager, Health Policy & Quality Department; Julie Kauffman, Program Manager, Health Policy & Quality Department.
Appendix B: Disclosures and Conflicts of Interest (COIs)
SNMMI rigorously attempted to avoid any actual, perceived, or potential COIs that might have arisen as a result of an outside relationship or personal interest on the part of the workgroup members or external reviewers. Workgroup members were required to provide disclosure statements of all relationships that might be perceived as real or potential COIs. These statements were reviewed and discussed by the workgroup chair and SNMMI staff and were updated and reviewed by an objective third party at the beginning of every workgroup meeting or teleconference. The disclosures of the workgroup members can be found in Table 3. A COI was defined as a relationship with industry—including consulting, speaking, research, and nonresearch activities—that exceeds $5,000 in funding over the previous or upcoming 12- month period. In addition, if an external reviewer was either the principal investigator of a study or another key member of the study personnel, that person’s participation in the review was considered likely to present a COI. All reviewers were asked about any potential COI. A COI was also considered likely if an external reviewer or workgroup member was either the principal investigator or a key member of a study directly related to the content of this AUC. All external reviewers were asked about any potential COI.
Table 3. Relationships with Industry and Other Entities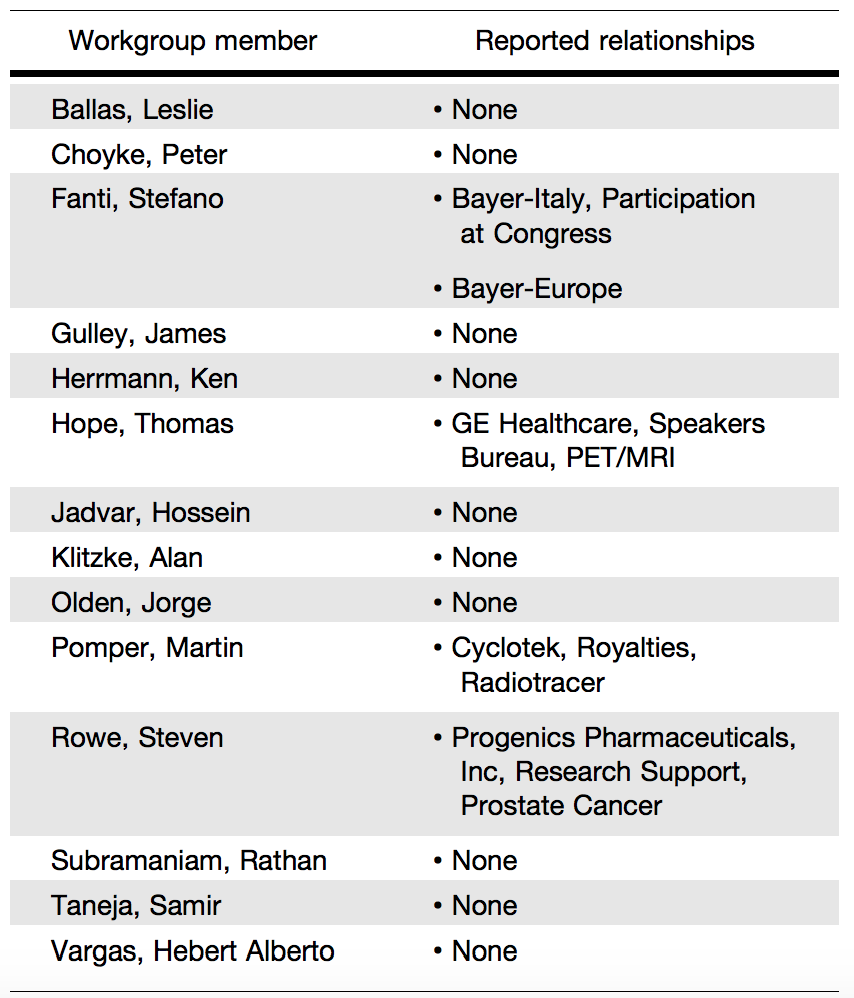
Appendix C: Public Commentary
The workgroup solicited information from all communities through the SNMMI website and through direct solicitation of SNMMI members. The comments and input helped to shape the development of these AUC on imaging evaluation of BCR of prostate cancer after definitive primary treatment.
Authors: Hossein Jadvar1, Leslie K. Ballas2, Peter L. Choyke3, Stefano Fanti4, James L. Gulley5, Ken Herrmann4, Thomas A. Hope1, Alan K. Klitzke6, Jorge D. Oldan1,3, Martin G. Pomper7, Steven P. Rowe1, Rathan M. Subramaniam6,8, Samir S. Taneja9, Herbert Alberto Vargas3, and Sukhjeet Ahuja1
1. Society of Nuclear Medicine and Molecular Imaging, Reston, Virginia
2. American Society for Radiation Oncology, Arlington, Virginia
3. American Society of Clinical Oncology, Alexandria, Virginia
4. European Association of Nuclear Medicine, Vienna, Austria
5. American College of Physicians, Philadelphia, Pennsylvania
6. American College of Nuclear Medicine, Reston, Virginia
7. World Molecular Imaging Society, Culver City, California
8. American College of Radiology, Reston, Virginia
9. American Urological Association, Linthicum Heights, Maryland
Published Date: May 2020
Related Content:
View or Download: Appropriate Use Criteria for Imaging Evaluation of Biochemical Recurrence of Prostate Cancer After Definitive Primary Treatment
View or Download: Condensed Fact sheet: Explanation of Appropriate Use Criteria (AUC) for Diagnostic Procedures


