External beam radiotherapy, along with radical prostatectomy, has been a mainstay treatment option for prostate cancer for decades and is currently recommended by numerous guidelines for the treatment of intermediate- and high-risk disease.1-3 Unlike surgery which is often, at least initially, planned as a monotherapy, many patients who opt for external beam radiotherapy will receive concurrent therapy. This center of excellence article will review the evidence for concurrent systemic therapy with radiotherapy in men with localized and locally advanced disease.
Androgen Deprivation Therapy
There have been several seminal trials that have established a survival benefit for the combination of androgen deprivation therapy plus radiotherapy among men with prostate cancer. In a trial of 415 patients with T1-2 WHO grade 3 or T3-4 N0-1 M0 disease randomized to radiotherapy vs radiotherapy plus 3 years of ADT, Bolla et al. showed that 5-year clinical disease-free survival was 40% (95% CI 32-48) in the radiotherapy-alone group and 74% (95% CI 67-81) in the combined-treatment group (p = 0.0001). Additionally, 5-year overall survival was 62% (95% CI 52-72) and 78% (95% CI 72-84), respectively (p = 0.0002) and 5-year specific survival 79% (95% CI: 72 – 86%) and 94% (95% CI: 90 – 98%).4 The PR-9 trial randomized 1,205 men with locally advanced or high-risk organ confined prostate cancer to receive lifelong ADT +/- radiotherapy. Over a median follow-up of 6.0 years (IQR 4.4 – 8.0), the addition of radiotherapy to ADT improved overall survival at 7 years (74%, 95% CI: 70 – 78% versus 66%, 95% CI: 60 – 70%; HR: 0.77, 95% CI: 0.61 - 0.98).5
Long-term follow-up of the D’Amico trial assessing ADT plus radiotherapy versus radiotherapy alone for patients with localized, unfavorable risk prostate cancer was published in 2015.6 This trial previously showed that addition of six months of ADT to radiotherapy increased overall survival among these men (radiotherapy vs radiotherapy + ADT: HR 1.8, 95% CI: 1.1 – 2.9, p = 0.01).7 After a median follow-up of 16.62 years (IQR: 15.42 to 17.67), 76% of men died, including 19% of prostate cancer, 25% of cardiac causes, and 56% from other causes. Additionally, this analysis showed that underlying comorbidity significantly modified the benefit of ADT: in patients with moderate or severe comorbidity, use of radiotherapy alone was associated with decreased cardiac mortality (HR: 0.17, 95% CI: 0.06 - 0.46) and overall mortality (HR: 0.36, 95% CI: 0.19 – 0.67), with no significant difference in non-prostate cancer mortality (HR: 2.79, 95% CI: 1.02 - 7.60). Conversely, in men with no or minimal comorbidity, radiotherapy alone was associated with an increased risk of overall mortality and prostate cancer mortality, without coinciding decreases in cardiac mortality or non-prostate cancer mortality. In 2016, the results of the EORTC 22991 randomized trial were published.8 This trial assessed if biochemical disease-free survival is improved by adding six months of androgen suppression to primary radiotherapy for intermediate- or high-risk localized prostate cancer. Among 819 patients, the median patient age was 70 years, 74.8% were intermediate risk and 24.8% were high risk. At 7.2 years median follow-up, radiotherapy plus androgen suppression significantly improved biochemical disease-free survival (HR: 0.52, 95% CI: 0.41 - 0.66), as well as clinical progression-free survival (HR: 0.63, 95% CI: 0.48 - 0.84).
Following these initial trials which demonstrated the survival benefit of the combination of ADT with radiotherapy, compared to radiotherapy alone, one of the key initiatives of recent radiotherapy trials has been delineating the appropriate dose/duration of ADT to give concurrently with radiotherapy. The DART01/05 GICOR trial was a randomized, controlled phase III trial assessing high-dose radiotherapy with short-term or long-term ADT for patients with T1c-T3b N0M0 prostate cancer with intermediate-risk and high-risk features. Patients were randomly assigned 1:1 to receive either four months of ADT combined with three-dimensional conformal radiotherapy at a minimum dose of 76 Gy (range 76-82 Gy; short-term ADT group) or the same treatment followed by 24 months of adjuvant ADT (long-term ADT group), stratified by prostate cancer risk group (intermediate risk vs high risk). In this Spanish multi-center trial, 178 patients were randomly assigned to receive short-term ADT and 177 to receive long-term ADT. After a median follow-up of 63 months (IQR 50 - 82), 5-year biochemical disease-free survival was significantly better among patients receiving long-term ADT than among those receiving short-term ADT: 90% (95% CI: 87 - 92) vs 81% (95% CI: 78 – 85%), HR: 1.88, 95% CI 1.12 - 3.15. Furthermore, the 5-year overall survival (95% vs 86%; HR: 2.48, 95% CI 1.31 - 4.68) and 5-year metastasis-free survival (94% vs 83%; HR: 2.31, 95% CI: 1.23 - 3.85) rates were also significantly better in the long-term ADT group than in the short-term ADT group. Not surprisingly, the authors noted that the effect of long-term ADT on biochemical disease-free survival, metastasis-free survival, and overall survival was more evident in patients with high-risk disease than in those with low-risk disease. However, it must be noted that the incidence of non-fatal cardiovascular events was higher in the long-term ADT group.9
Updated 10-year results presented at ASTRO 2021 however demonstrated no continued benefit for long-term ADT, with no significant differences in biochemical disease-free survival (70% vs 62%, HR 1.2, p = 0.40), metastasis-free survival (96% vs 84%, HR 1.1, p=0.06), or overall survival (78% vs 73%, HR 1.2, p = 0.51) noted. Although a greater absolute magnitude of difference was noted in the high-risk group (biochemical disease-free survival: 67% vs 54%; metastasis-free survival: 77% vs 65%; overall survival: 79% vs 67%), these comparisons were not statistically significant.
The prior standard ADT duration was 28-36 months when combined with radiotherapy for high-risk disease. As such, the TROG RADAR trial assessed whether the addition of 12 months of adjuvant ADT, 18 months of zoledronic acid, or both, can improve outcomes in men with locally advanced prostate cancer who receive six months of ADT and prostate radiotherapy.10 In 2019, this trial reported 10-year outcomes. There were 1,071 patients randomized to radiotherapy plus six months of ADT or radiotherapy plus 18 months of ADT. This trial found that 18 months of ADT plus radiotherapy was more effective in improving prostate cancer specific mortality for locally advanced prostate cancer compared to six months of ADT plus radiotherapy (HR 0.70, 95% CI: 0.50-0.98), but the addition of zoledronic acid to this treatment regimen is not beneficial:
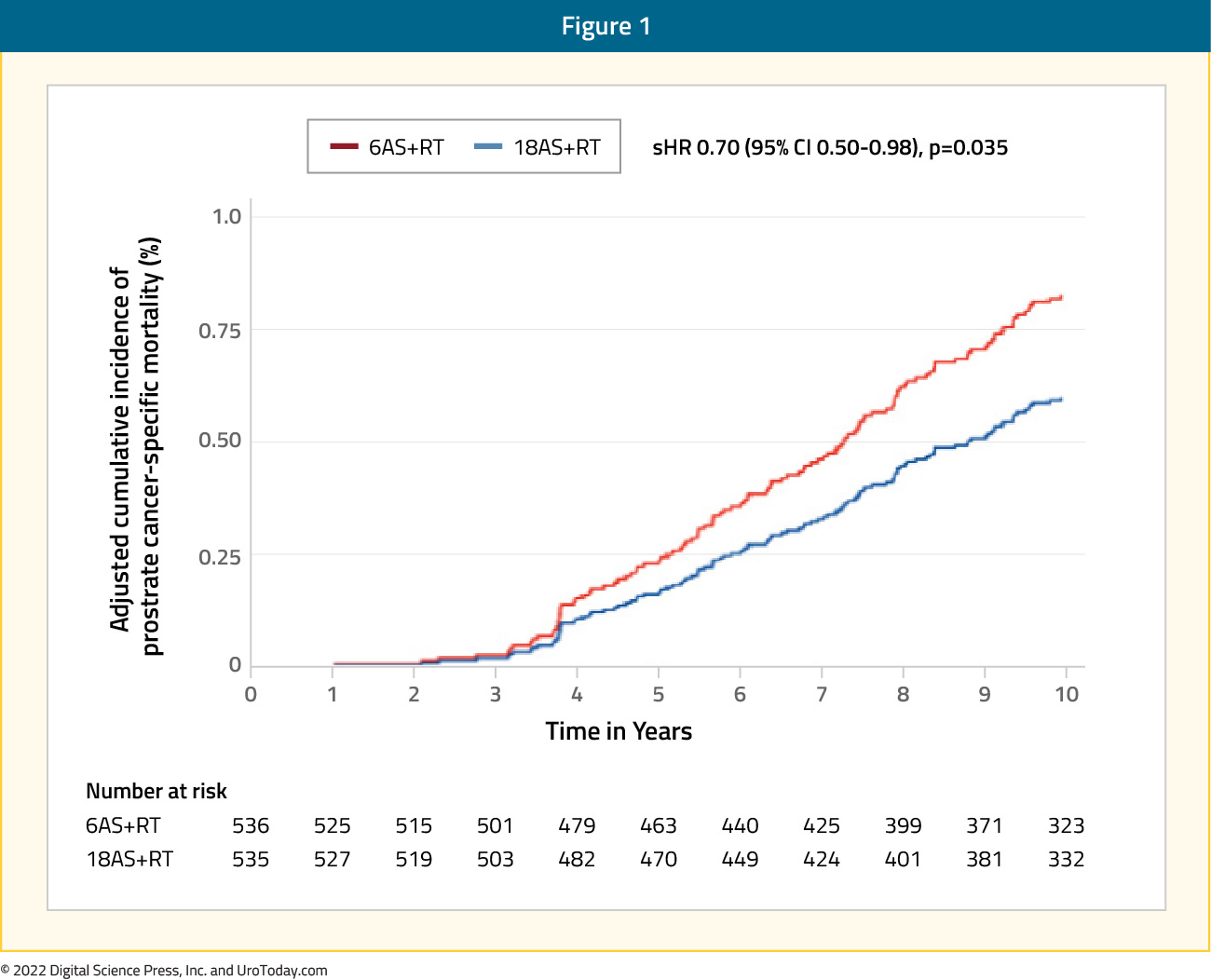
The PCS IV Trial randomized 630 patients with a median follow-up of 9.4 years to radiotherapy plus 18 months of ADT or radiotherapy plus 36 months of ADT.11 The 5-year OS rates were 91% for long term ADT arm (95% CI 88-95%) and 86% for short term ADT arm (95% CI: 83 - 90%, p=0.07). The quality of life analysis showed a significant difference (p<0.001) in six scales and 13 items favoring 18 months of ADT.
Despite level one evidence supporting use of concurrent androgen deprivation therapy for patients treated with radiation therapy, there remains an underutilization of concurrent androgen suppression in real-world practice. To address this, the MARCAP (Meta-Analysis of Randomized trials in CAncer of the Prostate) consortium was established. This consortium seeks to address these gaps by acting as a data repository for international trials groups to consolidate the results of randomized trial data.
In 2022, results of two MARCAP meta-analyses were published, the first of which was published in Lancet Oncology in February. This was a meta-analysis of 12 trials with individual patient data (n=10,583) that evaluated use or prolongation of ADT, either in the neoadjuvant (3-4 to 6-9 months) or adjuvant settings (4-6 to 18-36 months). Metastasis-free survival was the primary endpoint and all NCCN risk groups were included. After a median follow-up of 11.4 years (IQR: 9.0 – 15.0), the addition of androgen suppression to radiotherapy significantly improved metastasis-free survival (HR: 0.83; 95% CI: 0.77 – 0.89), as did adjuvant ADT prolongation (HR: 0.84; 95% CI: 0.78 – 0.91), but not neoadjuvant ADT extension (HR: 0.95; 95% CI: 0.83 – 1.09). Treatment effects were similar irrespective of radiotherapy dose, patient age, or NCCN risk group.12
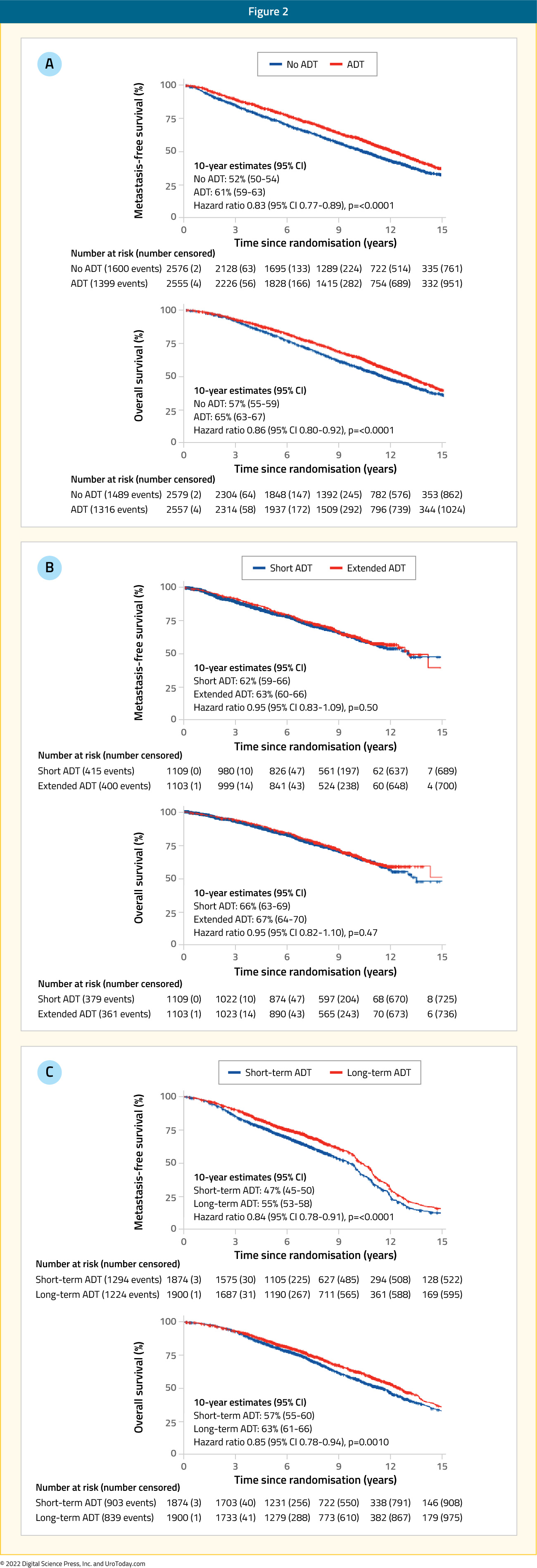
The next meta-analysis was published in European Urology in July 2022. This was a network meta-analysis of individual patient data from 13 multicenter randomized trials, including 11,862 patients, 45% of who were NCCN high-, 45% intermediate-, and 9% low-risk. In this analysis, low-dose radiotherapy was defined as 64 to <74 Gy and high-dose as ≥74 Gy. Androgen deprivation therapy duration was classified as short-term (3 to 6 months) or long-term (18 to 36 months). Patients were stratified into one of six treatment strategies:
- Low-dose radiotherapy
- Low-dose radiotherapy + short-term ADT
- Low-dose radiotherapy + long-term ADT
- High-dose radiotherapy
- High-dose radiotherapy + short-term ADT
- High-dose radiotherapy + long-term ADT
The pre-defined primary endpoint was metastasis-free survival. After a median follow-up of 8.8 years (IQR: 5.7 – 11.5), the highest metastasis-free survival rates were seen in patients treated with long-term ADT, irrespective of whether high (HR compared to low-dose RT alone: 0.56; 95% CI: 0.39 – 0.81) or low dose radiotherapy (HR: 0.60; 95% CI: 0.49 – 0.73) was given. Radiotherapy dose escalation did not improve metastasis-free survival either in the absence of ADT (HR: 0.97, 95% confidence interval: 0.80 - 1.18) or with short-term (HR: 0.99, 95% CI: 0.8 - 1.23) or long-term ADT (HR: 0.94, 95% CI: 0.65-1.37). According to P-score ranking and rankogram analysis, high-dose radiotherapy plus long-term ADT was the optimal treatment strategy for both biochemical recurrence-free survival and longer-term outcomes.13

Chemotherapy
Given the survival benefits seen with docetaxel in the metastatic hormone sensitive14,15 and resistant disease settings,16 the significant relapse rates seen with primary treatment alone for patients with high-risk prostate cancer,17 and non-overlapping toxicity profiles, docetaxel addition to radiation plus androgen deprivation therapy has been evaluated in high-risk prostate cancer patients.
Initial trials in this disease space failed to demonstrate an overall survival benefit to addition of chemotherapy to radiation in the non-metastatic prostate cancer patients (SPCG-13,18 GETUG-12).19 The SPCG-13 phase III trial randomized 376 patients with intermediate- or high-risk prostate cancer receiving “radical" radiotherapy with neoadjuvant/adjuvant ADT to either six cycles of adjuvant docetaxel (n=188) or surveillance (n=188). After a median follow-up of 59 months, this trial failed to meet its primary endpoint of biochemical disease-free survival (HR: 1.14, 95% CI: 0.79 – 1.64).18 While GETUG-12, a phase III trial of radiotherapy and ADT +/- docetaxel/estramustine in 413 high-risk localized prostate cancer patients (T3-T4, Gleason ≥8, PSA ≥20 ng/mL, pN +) met its primary endpoint of relapse-free survival (adjusted HR: 0.71, 95% CI: 0.54 – 0.94, p=0.017), no overall survival benefits were demonstrated.19
The first trial in this disease space to demonstrate an overall survival benefit was the NRG Oncology RTOG 0521 trial which was published in 2019 by Rosenthal et al. This multicenter randomized trial enrolled patients with high-risk nonmetastatic disease between 2005 and 2009. Patients (n=563) were randomly assigned to receive standard long-term ADT plus radiotherapy with or without adjuvant chemotherapy. Median PSA level in the cohort was 15.1 ng/ml, with 53% of patients having Gleason Score 9-10 disease, and 27% having cT3-4 disease. Over a median follow-up of 5.7 years, the 4-year overall survival rate was 89% (95% CI: 84% -92%) for ADT and radiotherapy, compared to 93% (95% CI: 90% - 96%) for ADT and radiotherapy plus chemotherapy (HR: 0.69, 90%: CI 0.49-0.97):
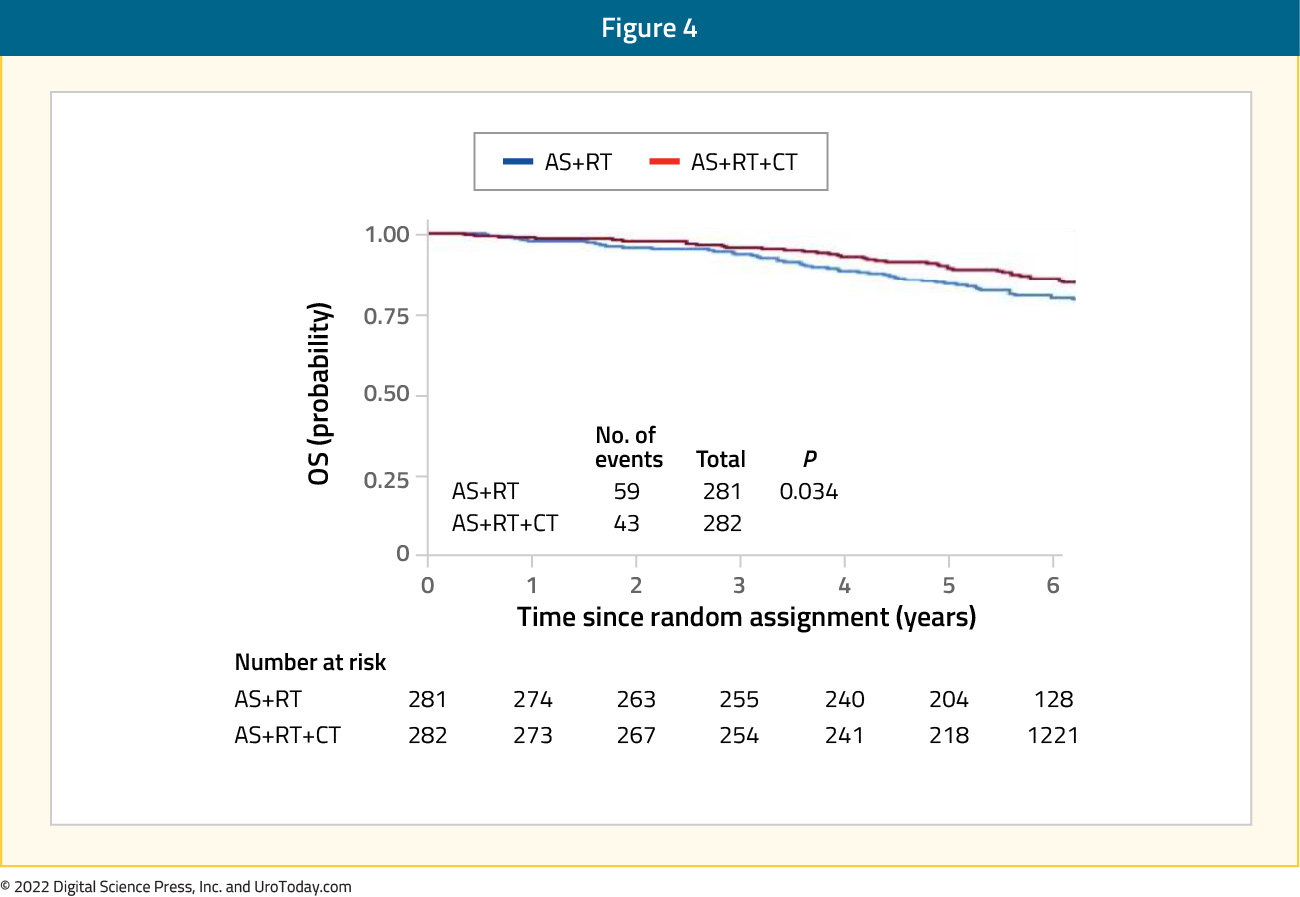
Six-year rates of distant metastases (9.1% versus 14%) and disease- free survival (65% versus 55%) also consistently favored the chemotherapy arm. However, although fewer prostate cancer-related deaths were seen in the chemotherapy arm (16 versus 23 deaths), there were no significant differences between the arms.20
It has been hypothesized that an overall survival benefit, without a concurrent significant improvement in prostate cancer-related mortality, may be secondary to a reduced incidence of radiotherapy-induced cancers/mortality in patients receiving docetaxel. To evaluate this hypothesis, D’Amico et al. conducted an international phase III trial of radiation and androgen deprivation therapy plus docetaxel (60 mg/m2 once every 3 weeks for three cycles before radiotherapy and 20 mg/m2 once weekly during radiotherapy) versus radiation/androgen deprivation therapy in 350 men with T1c-4N0M0 unfavorable-risk prostate cancer. After a median follow-up of 10.2 years, no significant differences in overall survival were noted: the restricted mean survival time over 10 years was 9.11 versus 8.82 years; p=0.22). Interestingly, significantly fewer radiotherapy-induced cancers were observed in the chemotherapy arm (10-year estimates: 0.61% versus 4.90%; age-adjusted HR: 0.13; 95% CI: 0.02 – 0.97, p=0.046).21
Androgen Receptor Axis Targeted Therapies
Although addition of systemic treatment in the form of androgen receptor axis-targeted therapies (ARAT) to radiation and androgen deprivation may be seen as adding to the increasing treatment burden of high-risk patients, early addition of systemic treatment may paradoxically allow for subsequent treatment de-intensification and, thus, decreased toxicity. Interim results of the AASUR were presented at ASCO 2021. This was a phase II trial of 46 patients with nonmetastatic, very high-risk prostate cancer, defined as Gleason Score 9-10, >4 cores of Gleason Score 9 disease, or 2 high-risk features (including cT3/T4 disease) who were treated with ultrahypofractionated radiotherapy (7.5 – 8.0 Gy x 5 fractions), six months of ADT (as opposed to the standard 18-36 months), apalutamide and abiraterone acetate plus prednisone. The primary endpoint was biochemical recurrence, as per Phoenix criteria (nadir PSA + 2ng/ml). The 3-year biochemical recurrence-free survival was 89.7% (95% CI: 81.0 – 99.3). The median time to testosterone recovery (i.e. non-castrate) was 6.5 months (range: 2.5 – 25.5). Grade 3 toxicities or worse were seen in 15 patients: 12 (18.8%) with hypertension and 3 (4.7%) with rash. Evaluations using the EPIC-26 score demonstrated no decline in urinary or bowel domains, although sexual (-11.9) and hormone (-5.7) domains showed a significant decline.22
Combined results from two phase III trials (STAMPEDE arms G and J) were recently published in The Lancet in 2022. These arms of the STAMPEDE trial were designed to assess the benefit of ARAT addition to standard of care treatment for men with high-risk non-metastatic prostate cancer, as per conventional imaging, defined as:
- Node positive, OR
- If node negative:
- High-risk, defined by at least two of the following:
- cT3-4
- Gleason Score 8-10
- PSA ≥40 ng/mL
- High-risk, defined by at least two of the following:
OR
- Relapsing with high-risk features:
- ≤12 months of total ADT with an interval of ≥12 months without treatment and PSA concentration ≥4 ng/mL with a doubling time of <6 months, OR
- PSA ≥20ng/mL
Local radiotherapy to the prostate and seminal vesicles (74 Gy in 37 fractions or equivalent using hypofractionation) was mandated for node negative and encourage for node positive disease (85% of total cohort planned for radiotherapy). In the first trial, patients were randomized 1:1 to either androgen deprivation therapy alone or with abiraterone acetate 1000 mg and prednisone 5 mg daily. In the second trial, patients received either androgen deprivation therapy alone (control arm consisted of separate patient cohort to those in the 1st trial) or with both abiraterone/prednisone and enzalutamide 160 mg daily. Androgen suppression was given for 3 years and combination therapy for 2 years, except if local radiotherapy was omitted when treatment could be delivered until progression. The primary endpoint of this meta-analysis was metastasis-free survival. Secondary endpoints were overall survival, prostate cancer-specific survival, biochemical failure-free survival, progression-free survival, and toxicity and adverse events.
Between 2011 and 2016, 1974 patients were recruited with 914 patients in the first trial (combination arm: 459, control arm: 455) and 1060 patients in the second trial (combination arm: 527, control arm 533). Median age was 68 years (IQR: 63 – 73), median PSA was 34 ng/ml (IQR: 14.7 – 47), and 39% of patients were node positive. After a median follow-up of 72 months, combined analysis of the two trials demonstrated superior metastasis-free survival in the combination arms (HR: 0.53, 95% CI: 0.44 – 0.64, p < 0.0001), with 6-year metastasis-free survival of 82% (95% CI: 79 – 85%) and 69% (66 – 72%), respectively:
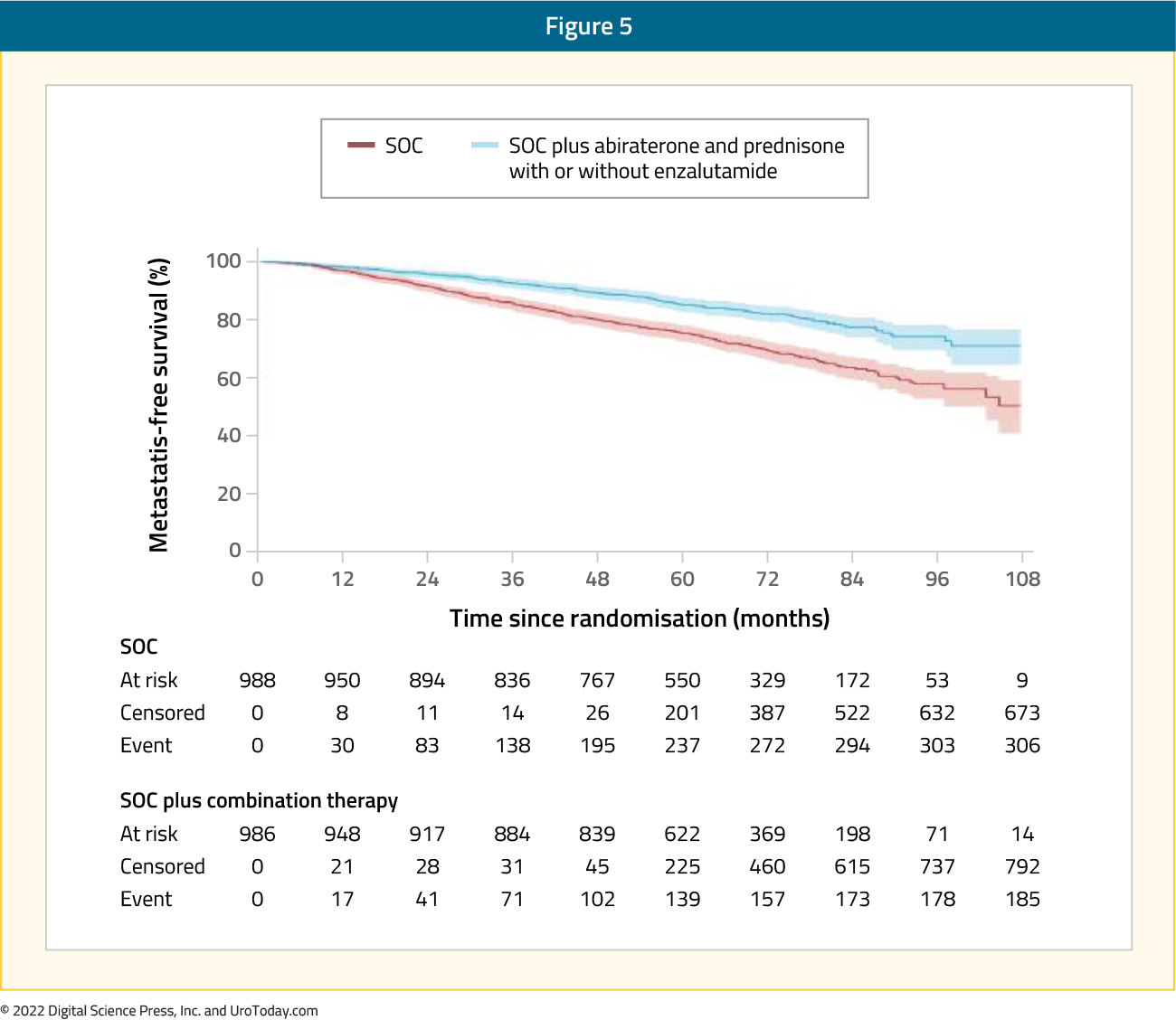
There was no evidence of a difference in metastasis-free survival when enzalutamide and abiraterone acetate were administered concurrently compared with abiraterone acetate alone (interaction HR 1.02, 0.70-1.50, p=0.91) and no evidence of between-trial heterogeneity (I2 p=0.90). Secondary outcomes of overall survival (HR: 0.60, 95% CI: 0.48 – 0.73), prostate cancer-specific survival (HR: 0.49, 95% CI: 0.37 – 0.65), biochemical failure-free survival (HR: 0.39, 95% CI: 0.33 – 0.47), and progression-free survival (HR: 0.44, 95% CI: 0.36 – 0.54) were all superior in the combination arms. Grade 3 or worse adverse events within the first 24 months were more common in the combination arms (37% versus 29% in the abiraterone trial; 58% versus 32% in the abiraterone + enzalutamide trial). The two most frequent adverse events were hypertension (abiraterone trial: 5% versus 1%; abiraterone + enzalutamide: 14% versus 2%) and alanine transaminitis (abiraterone trial: 6% versus <1%; abiraterone + enzalutamide: 13% versus 1%). Thus, among men with high-risk non-metastatic prostate cancer, combination therapy is associated with significantly higher rates of metastasis-free survival compared with androgen suppression alone. As such, abiraterone acetate with prednisolone should be considered a new standard treatment for this population.23
Risk Stratification Using Genomic Classifiers and Basal-Luminal Subtyping
Given some of the highlighted uncertainties regarding the optimal use of treatment intensification, as well as considerations related to the balance of oncologic benefit and toxicity, there has been considerable interest in better risk stratifying patients to allow for more personalized treatment allocation. Risk stratification using the Decipher® genomic classifier has recently been carried out as a combined ad hoc analysis of three trials (NRG Oncology/RTOG 9202, 9413, and 9902) to help distinguish which patients with high-risk prostate cancer would benefit from early treatment intensification versus not. In brief, the NRG/RTOG 9202 was a phase 3 trial of the use of long-term total androgen suppression (flutamide + zoladex) following neoadjuvant hormonal cytoreduction and radiotherapy in locally advanced carcinoma of the prostate. Patients were included if they were T2c-T4, PSA <150 ng/mL, N0 (N1 only below the iliac lines), M0. Radiotherapy included a total of 65-70 Gy for stage T2c (regional lymphatics only 44-46 Gy with 20-29 boost to the prostate) and a total of 67.5-70 Gy for stage T3 and T4. NRG/RTOG 9902 was a phase 3 protocol of androgen suppression and radiation therapy versus androgen suppression and radiotherapy followed by chemotherapy with paclitaxel, estramustine, and etoposide for localized, high-risk, prostate cancer. Patients were included if they had a PSA of 20-100 ng/mL and Gleason Score > 7 (any T stage) or clinical stage > T2 and Gleason Score > 8 (PSA <= 100) (M0, Nx or N0). Radiotherapy was 70.2 Gy (regional lymphatics only 46.8 Gy with 23.4 Gy boost to the prostate). NRG/RTOG 9413 was a phase 3 trial comparing whole pelvic irradiation followed by a conedown boost to boost irradiation only and comparing neoadjuvant to adjuvant total androgen suppression. Patients were included if they were T2c-T4, Gleason Score 6+, PSA < 100 ng/mL, >15% risk of lymph node positivity (based on the Partin’s table), M0, and N1 must be below the pelvis proper. Total androgen suppression included flutamide and Zoladex or Lupron, and radiotherapy included 70.2 Gy (Arms 1 and 3: whole pelvic irradiation; Arms 2 and 4: prostate only). Pre-treatment biopsy samples were used for this analysis.
In aggregate analysis relying on these three trials, on multivariable analysis, the genomic classifier was prognostic for distant metastasis (HR: 1.24, 95% CI: 1.11 - 1.39), prostate-cancer specific mortality (HR: 1.27, 95% CI: 1.13 - 1.43), and overall survival (HR: 1.12, 95% CI: 1.05 - 1.20). Stratified by low (genomic classifier <0.23), intermediate (genomic classifier 0.23-0.32), and high (genomic classifier > 0.32) risk groups, the cumulative incidences of outcomes by these genomic classifier risk groups was statistically significant for distant metastases (p < 0.001), prostate cancer specific mortality (p < 0.001), and OS (p = 0.002).24 These findings have served as the foundation for the NRG GU-009/PREDICT-RT trial that will assess the duration of androgen deprivation therapy and role of treatment intensification with apalutamide in NCCN high-risk prostate cancer patients:

Using patient biopsy samples from the same three trials (NRG Oncology/RTOG 9202, 9413, and 9902), the authors similarly evaluated the prognostic and predictive ability of a 215 gene basal-luminal prostate subtyping classifier to predict biochemical failure, distant metastasis, metastasis-free survival, prostate cancer-specific mortality, and overall survival. Of 265 samples, 40% were basal and 60% were luminal. In a multivariable analysis, the basal subtype had a worse prognosis compared to luminal for nearly all endpoints including metastasis-free survival (HR: 1.8, p<0.001), PCSM (HR: 2.8, p<0.001), and OS (HR: 1.8, p<0.001). In absolute terms, prostate cancers-specific mortality rates at 10 years were 26% (95% CI: 17 - 35%) for basal versus 11% (95% CI: 6 - 15%) for luminal subtypes.
Notably, a significant interaction between subtype (basal versus luminal) and androgen suppression duration was observed for both biochemical failure (p = 0.02) and prostate cancer mortality (p = 0.007). Basal subtypes significantly benefitted from long-term versus standard androgen suppression (10-year PCSM 5% [95% CI 0-11%] vs 42% [29-56%], p<0.001), while luminal outcomes did not differ by ADT duration (p = 0.72). As such, the authors concluded that basal versus luminal subtype may allow for personalization of androgen suppression use and duration when used concurrently with radiotherapy for patients with high-risk prostate cancer.
Conclusions
For many patients opting for external beam radiotherapy for localized or locally advanced prostate cancer, concurrent androgen deprivation therapy is a key component of the treatment paradigm. Numerous RCTs have helped to delineate the optimal duration of therapy, in a risk stratified manner. Ongoing work seeks to further personalize care with the use of molecular markers.
Written by:
- Rashid Sayyid, MD MSc, University of Toronto, Toronto, ON
- Zachary Klaassen, MD MSc, Medical College of Georgia, Augusta, Georgia, USA
Published: January 2023


