Introduction
Prostate cancer, while commonly diagnosed in early forms, remains the second leading cause of cancer mortality in the United States and Europe.1 For patients who die of prostate cancer, some will be initially diagnosed and treated for metastatic castration-sensitive disease (mCSPC) while others will progress through non-metastatic castration-resistant disease (nmCPRC) following initial local therapy followed by androgen deprivation therapy (ADT) for biochemical recurrence. In either case, nearly all men who die of prostate cancer will have metastatic castration-resistant disease (mCRPC) prior to death.
In the past 15 years, the treatment of patients with advanced prostate cancer has been revolutionized with the introduction of no less than nine agents with proven overall survival benefits. This article will narratively review the evidence underpinning recent changes in the management of patients with mCRPC, mCSPC, and nmCRPC.
Metastatic Castration-Resistant Prostate Cancer (MCRPC)
The last 10 years
The first major advances leading to survival gains in systemic therapy for patients with advanced prostate cancer began with the introduction of docetaxel for men with castration-resistant disease in 2004.¹ Since that time, there has been considerable progress in the development of treatment options and improved survival for men with mCRPC.² In patients with mCRPC, the use of abiraterone acetate plus prednisone and enzalutamide both prior to chemotherapy3-6 and following prior docetaxel chemotherapy7,8 have demonstrated significant survival benefits (in the range of 4-month median survival benefit). Among men who had previously received docetaxel for mCRPC, treatment with cabazitaxel, as compared to mitoxantrone, was associated with significant survival benefits.9 In a similar timeframe, the IMPACT study, Provenge® (Sipuleucel-T) Active Cellular Immunotherapy Treatment of Metastatic Prostate Cancer After Failing Hormone Therapy (IMPACT), demonstrated approximately 20% improved overall survival (OS) for patients receiving sipuleucel-T,10 though this approach has not been widely adopted.
The FIRSTANA trial, Cabazitaxel Versus Docetaxel Both With Prednisone in Patients With Metastatic Castration-Resistant Prostate Cancer (FIRSTANA), compared docetaxel and two doses of cabazitaxel in chemotherapy naïve patients with mCRPC. Overall, no large differences between the regimes were seen with respect to oncologic efficacy or tolerability.11
In the phase 3 ALSYMPCA trial, A Phase III Study of Radium-223 Dichloride in Patients With Symptomatic Hormone Refractory Prostate Cancer With Skeletal Metastases (ALSYMPCA), demonstrated significantly prolonged overall survival in patients who had castration-resistant prostate cancer and bone metastases, with a 30% reduction in the risk of death, as compared with placebo. In the updated analysis, the median survival was longer among patients who received radium-223 than among those who received placebo, by 3.6 months. All main secondary efficacy endpoints were significant and favored treatment with radium-223, including the clinically defined endpoint of the time to the first symptomatic skeletal event, which was significantly prolonged among patients who received radium-223.12
Recent advances
Significant progress continues to be made in the care of patients with mCRPC, including both a better understanding of administering currently approved medications and the introduction of new therapies.
The question of rationale drug sequencing, both in the mCRPC setting and including the use of novel agents in the mCSPC and nmCRPC settings, relates in large part to the issue of acquired resistance to each agent and the potential for cross-resistance. In data presented at ASCO 2018, Kim Chi, MD, and colleagues demonstrated that the sequence of abiraterone acetate plus prednisone followed by enzalutamide had significantly longer and deeper responses to second line therapy than those who received enzalutamide followed by abiraterone acetate plus prednisone, in a phase II randomized controlled trial.13
In contrast to this strategy of sequential androgen axis inhibition, the CARD trial, Cabazitaxel Versus the Switch to Alternative AR-targeted Agent (Enzalutamide or Abiraterone) in Metastatic Castration-resistant Prostate Cancer (mCRPC) Patients Previously Treated With Docetaxel and Who Rapidly Failed a Prior AR-targeted Agent (CARD), randomized 255 patients who had previously received both docetaxel and an androgen axis targeting agent (either abiraterone acetate plus prednisone or enzalutamide) to either cabazitaxel or the other androgen axis targeting agent.14 The primary outcome of interest was imaging-based progression-free survival with overall survival, response and toxicity considered secondarily. Over a median follow-up of 9.2 months, patients who receive cabazitaxel had a significantly lower risk of imaging-based progression or death (95 of 129, 74%; median progression-free survival (PFS) 8 months) compared to those who underwent androgen axis inhibitor switch (101 of 126, 80%; median PFS 3.7 months) (hazard ratio 0.54, 95% confidence interval 0.40 to 0.73). Overall survival was similarly improved with the use of cabazitaxel (13.6 months vs 11.0 months; hazard ratio 0.64, 95% confidence interval 0.46 to 0.89). Adverse events were in keeping with previously identified safety profiles and adverse events ≥ Grade 3 occurred in 56% of patients receiving cabazitaxel and 52% of those receiving androgen axis targeting agents. There are other ongoing trials assessing treatment sequencing, including PRIMCAB (NCT02379390), Cabazitaxel Versus the Switch to Alternative AR Targeted Therapy Enzalutamide or Abiraterone in Metastatic Castration-Resistant Prostate Cancer (mCRPC) Primary Resistant Patients to Abiraterone or Enzalutamide (PRIMCAB), and assessing whether combined therapy may overcome treatment resistance (NCT01949337), Enzalutamide With or Without Abiraterone and Prednisone in Treating Patients With Castration-Resistant Metastatic Prostate Cancer.
While cytotoxic chemotherapy and androgen axis targeting agents have proven beneficial, considerations for more targeted therapy have also proven attractive. While underlying dominant driving mutations are not widespread in prostate cancer, there have been a number of key genomic mutations that have been consistently identified in prostate cancer patients, across the disease spectrum including gene fusion/chromosomal rearrangements The transmembrane protease serine 2:v-ets erythroblastosis virus E26 oncogene homolog (TMPRSS2:ERG), androgen receptor (AR) amplification, inactivation of tumor suppressor genes (PTEN/PI3-K/AKT/mTOR, TP53, Rb1) and oncogene activation (c-MYC, RAS-RAF).15 More significantly, defects in DNA repair (DDR mutations) appear to be central in increasing one’s susceptibility to malignant transformation. The poly (adenosine diphosphate [ADP]-ribose) polymerase (PARP) enzyme and BRCA 1/2 (BReast CAncer gene 1 and 2) gene products play important roles in this process.16,17 In patients with DDR mutations, the United States Food and Drug Administration (FDA) has recently approved two PARP inhibitors, rucaparib and olaparib.18,19
The first PARP inhibitor to be approved by the U.S. FDA was rucaparib. On May 15, 2020, rucaparib was granted accelerated approval for patients with mCRPC and BRCA mutations (germline or somatic) who had progressed following treatment with androgen-axis targeted treatment and taxane-based chemotherapy, on the basis of the phase II TRITON2 trial, A Study of Rucaparib in Patients With Metastatic Castration-resistant Prostate Cancer and Homologous Recombination Gene Deficiency (TRITON2).21 TRITON2 is an international, multi-center, open-label phase II study which enrolled men with mCRPC who had disease progression following an androgen axis inhibitor and at least one taxane-based chemotherapy, and one of 13 associated homologous recombination repair gene alterations. Initial results were presented at ESMO 201821 and updated at ESMO 2019 at which time median follow-up was 13 months (range 4-29 months).22 For the patients with BRCA1/2 alteration, there was a 44% confirmed overall response rate and 52% confirmed prostate-specific antigen response rate, with durable responses demonstrated in the majority of responders. The FDA accelerated approval of rucaparib is contingent upon ongoing assessment in confirmatory trials, namely the phase III TRITON3 trial, A Study of Rucaparib Versus Physician’s Choice of Therapy in Patients With Metastatic Castration-resistant Prostate Cancer and Homologous Recombination Gene Deficiency (TRITON3), which is randomizing patients with mCRPC and mutations in BRCA1, BRCA2, or Ataxia-Telangiectasia mutated (ATM) to rucaparib or investigators choice of abiraterone acetate plus prednisone, enzalutamide, or docetaxel following progression on an androgen axis inhibitor.
On May 19, 2020, the FDA approved olaparib for the treatment of patients with germline or somatic homologous recombination repair gene-mutated mCRPC who progressed following treatment with enzalutamide or abiraterone acetate plus prednisone, on the basis of phase III PROfound randomized controlled trial, Study of Olaparib (Lynparza™) Versus Enzalutamide or Abiraterone Acetate in Men With Metastatic Castration-Resistant Prostate Cancer (PROfound Study).19 To be eligible for inclusion, men must have had alterations in one of 15 pre-specified genes involved in homologous recombination repair (BRCA 1/2, ATM, BRIP1, BARD1, CDK12, CHEK 1/2, FANCL, PALB2, PPP2R2A, RAD51B, RAD51C, RAD51D, RAD54L). The trialists then used biomarker-driven stratification to derive two study cohorts: Cohort A had alterations in BRCA1, BRCA2, or ATM while Cohort B had alterations in any of the other 15 included genes. In both patient cohorts, patients were randomized 2:1 to olaparib versus abiraterone acetate plus prednisone or enzalutamide. There was significantly improved progression-free survival in patients with mutations of BRCA1, BRCA2, or ATM (hazard ratio 0.34, 95% confidence interval 0.25 to 0.47). Similar results were seen in the combined cohort (hazard ratio 0.49, 95% confidence interval 0.38 to 0.63). Subgroup analysis showed similar results when stratified according to prior taxane use, measurable disease at baseline, location of metastasis (bone only, visceral, or other), performance status, age at randomization, region, and baseline PSA (dichotomized around the median). While data is immature (data maturity 38%), there is a suggestion of improved overall survival in Cohort A (hazard ratio 0.64, 95% confidence interval 0.43 to 0.97, p=0.02 with an alpha significance threshold of 0.01 based on alpha-spending function).
Adverse events were common in both patients on olaparib (any = 95%, grade ≥ 3 = 51%) and in the control group (any = 88%, grade ≥ 3 = 38%).20 The PROpel trial, Study on Olaparib Plus Abiraterone as First-line Therapy in Men With Metastatic Castration-resistant Prostate Cancer (PROpel), is currently randomizing patients with mCRPC to first-line treatment with olaparib + abiraterone acetate plus prednisone versus abiraterone acetate plus prednisone alone, without biomarker selection. Further, KEYNOTE-365, Study of Pembrolizumab Combination Therapies in Metastatic Castration-Resistant Prostate Cancer (KEYNOTE-365), Cohort A is testing pembrolizumab + Olaparib23 while KEYLYNK-010, Study of Pembrolizumab Plus Olaparib Versus Abiraterone Acetate or Enzalutamide in Metastatic Castration-resistant Prostate Cancer (mCRPC) (KEYLYNK-010), is randomizing 780 patients to pembrolizumab + olaparib versus abiraterone acetate plus prednisone or enzalutamide in patients with mCRPC.
Two other PARP inhibitors, talazoparib and niraparib, are currently under investigation. The TALAPRO1 trial, A Study of Talazoparib in Men With DNA Repair Defects and Metastatic Castration-Resistant Prostate Cancer (TALAPRO1), will assess talazoparib in patients with progressive disease following both taxane and androgen axis inhibitor treatment (and biomarker selection for DDR mutations likely to sensitize to PARP inhibitor) while TALAPRO2, Talazoparib + Enzalutamide vs. Enzalutamide Monotherapy in mCRPC (TALAPRO-2), will assess talazoparib + enzalutamide versus enzalutamide alone in first-line treatment of mCRPC without biomarker selection. The MAGNITUDE trial, A Study of Niraparib in Combination With Abiraterone Acetate and Prednisone Versus Abiraterone Acetate and Prednisone for Treatment of Participants With Metastatic Prostate Cancer (MAGNITUDE), is recruiting patients with mCRPC to first-line therapy with niraparib + abiraterone acetate plus prednisone or abiraterone acetate plus prednisone alone. Analysis is stratified by the presence (cohort A) or absence (cohort B) of DDR mutations.
While these studies have utilized PARP inhibitor monotherapy, combination therapy has also been assessed. In 2018, results of a phase 2 trial assessing olaparib with abiraterone acetate plus prednisone were published in Lancet Oncology.24 Among 142 patients randomized, median rPFS was significantly longer among those randomized to olaparib and abiraterone acetate plus prednisone (13.8 months, 95% confidence interval 10.8 to 20.4 months) than those randomized to abiraterone acetate plus prednisone alone (8.2 months, 95% confidence interval 5.5 to 9.7 months; hazard ratio 0.65, 95% confidence interval 0.44 to 0.97, p = 0.034). One treatment-related death (pneumonitis) occurred in the olaparib and abiraterone acetate plus prednisone group. As mentioned above, based on the results of this phase 2 study, the ongoing PROpel phase 3 trial will evaluate olaparib + abiraterone acetate plus prednisone in the first line mCRPC setting. Goal enrolment for PROpel is 720 patients, with an estimated trial completion of 2022.
Despite a promise in many tumor types, studies of immunotherapy utilizing checkpoint inhibitors have unfortunately proven generally unsuccessful to date in patients with advanced prostate cancer. In the phase III IMbassador250 trial, A Study of Atezolizumab (Anti-PD-L1 Antibody) in Combination With Enzalutamide in Participants With Metastatic Castration-Resistant Prostate Cancer (mCRPC) After Failure of an Androgen Synthesis Inhibitor And Failure of, Ineligibility For, or Refusal of a Taxane Regimen (IMbassador250), patients with mCRPC were randomized to enzalutamide alone or in combination with atezolizumab.25 Reported at the American Association for Cancer Research (AACR) virtual meeting 2020, there was no difference in overall survival between groups (hazard ratio 1.12, 95% confidence interval 0.91 to 1.37). COSMIC-021 is a phase Ib trial of cabozantinib with atezolizumab which had demonstrated promising results,26 prompting ongoing further investigation. In monotherapy, ipilimumab has failed to demonstrate a benefit in two trials,27,28 though there is more interest in combination therapy with nivolumab which is currently being examined in CheckMate-650: Study of Nivolumab Plus Ipilimumab, Ipilimumab or Cabazitaxel in Men With Metastatic Castration-Resistant Prostate Cancer (CheckMate 650). There are a number of other ongoing trials assessing nivolumab in combination with radium-223: (NCT04109729) Study of Nivolumab in Combination w Radium-223 in Men w Metastatic Castration-Resistant Prostate Cancer (Rad2Nivo), rucaparib: (NCT03572478) Rucaparib and Nivolumab in Patients With Prostate or Endometrial Cancer, and an experimental DNA vaccine: (pTVG-HP; NCT03600350) pTVG-HP and Nivolumab in Patients With Non-Metastatic PSA-Recurrent Prostate Cancer. In contrast to these negative data, pembrolizumab has received a tumor-agnostic approval for patients with MSI-high tumors, including prostate cancer. Ongoing trials including KEYNOTE-028: Study of Pembrolizumab in Participants With Advanced Solid Tumors (KEYNOTE-028), the multi-platform KEYNOTE-199: Study of Pembrolizumab in Participants With Metastatic Castration-Resistant Prostate Cancer (mCRPC) (KEYNOTE-199), KEYNOTE-365: Study of Pembrolizumab Combination Therapies in Metastatic Castration-Resistant Prostate Cancer (KEYNOTE-365), KEYNOTE-641: Study of Pembrolizumab Plus Enzalutamide Versus Placebo Plus Enzalutamide in Participants With Metastatic Castration-resistant Prostate Cancer (mCRPC) (KEYNOTE-641), KEYNOTE-921: Study of Pembrolizumab Plus Docetaxel Versus Placebo Plus Docetaxel in Chemotherapy-naïve Metastatic Castration-resistant Prostate Cancer (mCRPC) (KEYNOTE-921), and KEYLYNK-010: A Phase 3, Randomized Open-label Study of Pembrolizumab Plus Olaparib Versus Abiraterone Acetate or Enzalutamide in Participants With Metastatic Castration-resistant Prostate Cancer (mCRPC) Who Are Unselected for Homologous Recombination Repair Defects and Have Failed Prior Treatment With One Next-generation Hormonal Agent (NHA) and Chemotherapy (KEYLYNK-010). Each of these trials assessed pembrolizumab in combination with a variety of agents including enzalutamide, docetaxel, and olaparib in patients who had received various previous lines of therapy.
Finally, the field of theranostics is rapidly evolving. While PSMA-PET/CT has rapidly gained utilization as a diagnostic imaging modality, the use of the PSMA tracer allows for targeted radioligand therapy. Early single-center data have demonstrated promise for [177Lu]Lu-PSMA-617 (LuPSMA) as a treatment for mCRPC with promising PSA response rates and survival outcomes.30,31 The first prospective phase III trial VISION, Study of 177Lu-PSMA-617 In Metastatic Castrate-Resistant Prostate Cancer (VISION), radioligand therapy in patients with mCRPC who had progressed on prior therapy including both cytotoxic chemotherapy (docetaxel and cabazitaxel, or unwilling/unable to receive second-line cytotoxic chemotherapy) and androgen axis targeting agents have completed accrual of 815 patients who are randomized 2:1 to LuPSMA plus standard of care or standard of care alone with a primary outcome of overall survival (NCT03511664). The TheraP trial, A Trial of 177Lu-PSMA617 Theranostic Versus Cabazitaxel in Progressive Metastatic Castration-Resistant Prostate Cancer (TheraP), is a phase II trial randomizing men with progressive mCRPC to LuPSMA or cabazitaxel with a primary outcome of PSA response rate (NCT03392428).31 A summary timeline of recent FDA treatment approvals for mCRPC patients is as shown in Figure 1.
Metastatic Hormone-Sensitive Prostate Cancer
Following their introduction and evaluation in mCRPC, many agents have subsequently moved forward in the natural history of the disease including both into the metastatic castration-sensitive disease space (mCSPC) and non-metastatic castration resistance disease space (nmCRPC).
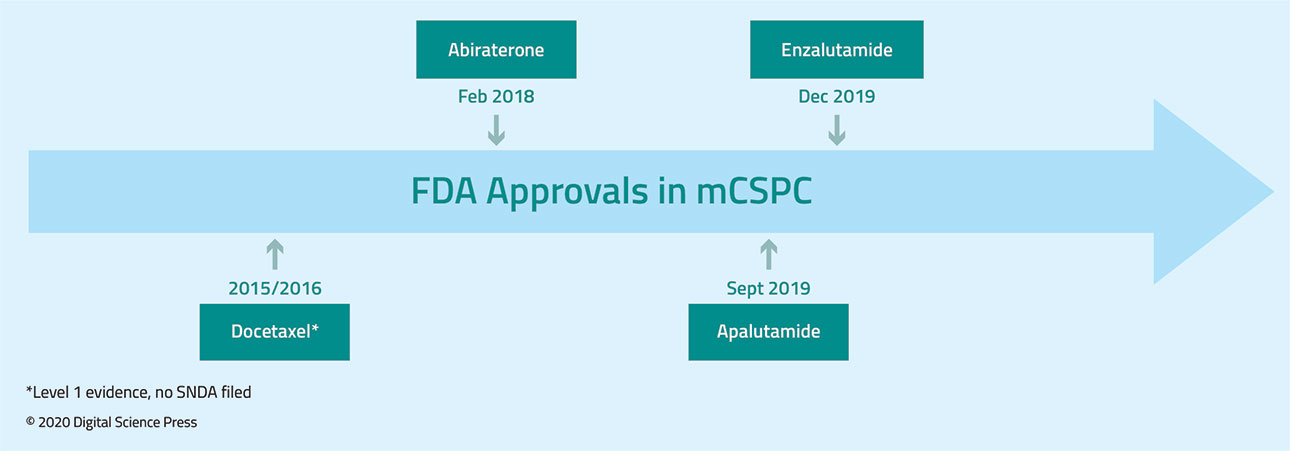
In the mCSPC disease space, randomized data support the use of docetaxel, abiraterone acetate plus prednisone, enzalutamide, and apalutamide. The first of these to be assessed was docetaxel. While the GETUG-15 trial, Androgen deprivation therapy (ADT) plus docetaxel (D) versus ADT alone for hormone-naïve metastatic prostate cancer (PCa) (GETUG-15), initially published in February 2013,32 failed to demonstrate a survival benefit to the addition of docetaxel to ADT in men with mCSPC (hazard ratio 0.88, 95% confidence interval 0.68 to 1.14), subsequent data from the CHAARTED trial, "ChemoHormonal Therapy Versus Androgen Ablation Randomized Trial for Extensive Disease in Prostate Cancer (CHAARTED), (hazard ratio 0.61, 95% confidence interval 0.47 to 0.80) and the first analysis of the multi-stage STAMPEDE trial, Systemic Therapy in Advancing or Metastatic Prostate Cancer: Evaluation of Drug Efficacy: A Multi-Stage Multi-Arm Randomised Controlled Trial (STAMPEDE), (hazard ratio 0.80, 95% confidence interval 0.65 to 0.99) both demonstrated significantly improved survival among men receiving docetaxel with a median survival difference of more than 12 months. Subsequent meta-analysis of these data demonstrated a statistically significant improvement in survival with the addition of docetaxel to ADT (pooled hazard ratio 0.72, 95% confidence interval 0.60 to 0.90).33 Longer-term follow-up of the CHAARTED trial suggested that this benefit is restricted to patients with high volume disease, defined as the presence of visceral metastases and/or greater than or equal to 4 bone lesions with at least one beyond the spine and pelvis.34
Following the publication of the data on docetaxel,33 the LATITUDE trial, A Randomized, Double-blind, Comparative Study of Abiraterone Acetate Plus Low-Dose Prednisone Plus Androgen Deprivation Therapy (ADT) Versus ADT Alone in Newly Diagnosed Subjects With High-Risk, Metastatic Hormone-naive Prostate Cancer (mHNPC), (LATITUDE), and STAMPEDE arm G were utilized to assess the role of abiraterone acetate plus prednisone in this disease space. Published concurrently, there was a significant improvement in overall survival for men randomized to receive abiraterone acetate plus prednisone, with the relative benefit of 37% in STAMPEDE (hazard ratio 0.63, 95% confidence interval 0.52 to 0.76)35 and 38% in LATITUDE (hazard ratio 0.62, 95% confidence interval 0.51 to 0.76).36
In 2019, we saw the emergence of data for enzalutamide in patients with mCSPC. The ARCHES trial, A Study of Enzalutamide Plus Androgen Deprivation Therapy (ADT) Versus Placebo Plus ADT in Patients With Metastatic Hormone Sensitive Prostate Cancer (mHSPC) (ARCHES), was powered to assess radiographic progression-free survival while ENZAMET, Enzalutamide in First Line Androgen Deprivation Therapy for Metastatic Prostate Cancer, (ENZAMET), assessed overall survival as its primary outcome. In the ARCHES trial, patients with predominately high-volume disease were randomized to enzalutamide + ADT or ADT alone. Notably, 17% of the cohort had previously received docetaxel. While overall survival data from this cohort is immature, the addition of enzalutamide was found to reduce the risk of radiographic progression versus ADT plus placebo by 61% (hazard ratio 0.39, 95% confidence interval 0.30 to 0.50).37 In contrast to the studies described above, ENZAMET utilized an active comparator and randomized men to enzalutamide or a standard first-generation anti-androgen in addition to ADT. Interim analysis after a median follow up of 34 months showed a significant survival benefit in the enzalutamide group versus those receiving standard nonsteroidal anti-androgens (hazard ratio 0.67, 95% confidence interval 0.52 to 0.86).38
Finally, the TITAN trial, A Study of Apalutamide Plus Androgen Deprivation Therapy (ADT) Versus ADT in Participants With mHSPC (TITAN), also published in 2019, provided randomized data for apalutamide in this disease space. As with the ENZAMET and ARCHES trials, patients were eligible if their have previously received docetaxel. Interim analysis at 24 months demonstrated significantly improved survival in men receiving apalutamide (hazard ratio 0.67, 95% confidence interval 0.51 to 0.89).39 Each of these agents has now been FDA approved for patients with mCSPC and are listed as category 1 recommendations within the NCCN guidelines.40 While there are no direct randomized comparative data, a number of comparative network meta-analyses have been performed.41,43 The most recent of these, from Marchioni et al., suggests that abiraterone acetate plus prednisone, enzalutamide, and apalutamide have superior overall survival when compared to docetaxel. Assessing adverse events, the authors found lower rates with abiraterone acetate plus prednisone and enzalutamide compared to docetaxel.43 Individual patient and physician preferences, longer-term sequencing strategies, local health systems factors and reimbursement, and considerations such as the current COVID-19 pandemic will likely have a significant influence on individual treatment choice.
There are numerous ongoing studies in this disease space including the ARASENS trial: ODM-201 in Addition to Standard ADT and Docetaxel in Metastatic Castration Sensitive Prostate Cancer (ARASENS), of darolutamide (ODM-201) which will include docetaxel in both arms, KEYNOTE-991: Efficacy and Safety of Pembrolizumab Plus Enzalutamide Plus Androgen Deprivation Therapy (ADT) Versus Placebo Plus Enzalutamide Plus ADT in Participants With Metastatic Hormone-Sensitive Prostate Cancer (mHSPC) (KEYNOTE-991) assessing pembrolizumab plus enzalutamide versus enzalutamide, TRIUMPH: Trial of Rucaparib in Patients With Metastatic Hormone-Sensitive Prostate Cancer Harboring Germline DNA Repair Gene Mutations (TRIUMPH) assessing rucaparib, a combined trial of docetaxel and PROSTVAC (vaccine) for Metastatic Castration-Sensitive Prostate Cancer, and Enzalutamide Plus Talazoparib for the Treatment of Hormone Sensitive Prostate Cancer (ZZ-First) assessing talazoparib plus enzalutamide. A summary timeline of FDA treatment approvals for mCSPC patients is as shown in Figure 2.
Non-Metastatic Castration-Resistant Prostate Cancer
The last disease space to see the influx of novel androgen axis targeting agents was nmCRPC. Since 2018, three new agents have been approved in this disease space: enzalutamide, apalutamide, and darolutamide.
While each agent was approved on the basis of demonstrated benefits in metastasis-free survival (MFS), recently presented data at the 2020 ASCO Virtual Annual Meeting from each trial has demonstrated improved overall survival (OS) benefit. Simultaneously published with the ASCO 2020 presentation, PROSPER, Safety and Efficacy Study of Enzalutamide in Patients With Nonmetastatic Castration-Resistant Prostate Cancer (PROSPER), demonstrated a significant improvement in overall survival for men with nmCRPC, PSA >2 ng/mL and a PSA doubling time <10 months who were randomized to enzalutamide, compared with placebo (hazard ratio 0.73, 95% confidence interval 0.61 to 0.89).44 While as yet unpublished, presented data from ARAMIS and SPARTAN demonstrated similar benefits from treatment with darolutamide (hazard ratio 0.69, 95% confidence interval 0.53 to 0.88) and apalutamide (hazard ratio 0.784, 95% confidence interval 0.64 to 0.96), respectively.
While a network meta-analysis of the updated overall survival data is inevitable and a randomized comparison of darolutamide and enzalutamide with a functional outcome endpoint is planned (DaroAct), comparative data to date are limited to network meta-analyses of metastasis-free survival and toxicity. Bayesian analysis demonstrates that apalutamide and enzalutamide are likely to maximize metastasis-free survival while darolutamide is the preferred agent with respect to toxicity.45 A summary timeline of FDA treatment approvals for nmCRPC patients is as shown in Figure 3.

Conclusions
After decades without the introduction of novel treatments with proven life-sustaining benefit, the field of advanced prostate cancer has been revolutionized over the past 15 years, and particularly over the last 5 years. While targeting the androgen axis continues to prove beneficial, novel approaches including PARP inhibitors to target mutations in DNA damage repair, immunotherapy, and theranostics offer alternate and exciting mechanisms to treat patients with advanced prostate cancer. Numerous ongoing studies will help to inform optimal combinations of these therapies as well as their sequencing.
While this article has focused on systemic therapy, there is interesting and emerging data on the benefit of treatment of the primary tumor in patients with low-volume systemic disease, including randomized data from STAMPEDE, Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE), a randomised controlled phase 3 trial (arm H)46 and HORRAD, the Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the Prostate in Patients with Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial (HORRAD).47
Written by: Christopher J.D. Wallis, MD PhD, Vanderbilt University Medical Center, Nashville, Tennessee, USA and Zachary Klaassen, MD MSc, Medical College of Georgia, Augusta, Georgia, USA
References:
- Tannock IF, de Wit R, Berry WR, et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N Eng J Med. 2004;351(15):1502-1512.
- Teo MY, Rathkopf DE, Kantoff P. Treatment of Advanced Prostate Cancer. Annu Rev Med. 2019;70:479-499.
- Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Eng J Med. 2013;368(2):138-148.
- Ryan CJ, Smith MR, Fizazi K, et al. Abiraterone acetate plus prednisone versus placebo plus prednisone in chemotherapy-naive men with metastatic castration-resistant prostate cancer (COU-AA-302): final overall survival analysis of a randomised, double-blind, placebo-controlled phase 3 study. Lancet Oncol. 2015;16(2):152-160.
- Beer TM, Armstrong AJ, Rathkopf DE, et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N Eng J Med. 2014;371(5):424-433.
- Beer TM, Armstrong AJ, Rathkopf D, et al. Enzalutamide in Men with Chemotherapy-naive Metastatic Castration-resistant Prostate Cancer: Extended Analysis of the Phase 3 PREVAIL Study. Eur Urol. 2017;71(2):151-154.
- de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Eng J Med. 2011;364(21):1995-2005.
- Scher HI, Fizazi K, Saad F, et al. Increased survival with enzalutamide in prostate cancer after chemotherapy. N Eng J Med. 2012;367(13):1187-1197.
- de Bono JS, Oudard S, Ozguroglu M, et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet Oncol. 2010;376(9747):1147-1154.
- Kantoff PW, Higano CS, Shore ND, et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Eng J Med. 2010;363(5):411-422.
- Oudard S, Fizazi K, Sengelov L, et al. Cabazitaxel Versus Docetaxel As First-Line Therapy for Patients With Metastatic Castration-Resistant Prostate Cancer: A Randomized Phase III Trial-FIRSTANA. J Clin Oncol. 2017;35(28):3189-3197.
- Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Eng J Med. 2013;369(3):213-223.
- Khalaf D, Annala M, Taavitsainen S, Finch D, Oja C, & Vergidis J, et al. Optimal sequencing of enzalutamide and abiraterone acetate plus prednisone in metastatic castration-resistant prostate cancer: a multicentre, randomised, open-label, phase 2, crossover trial. Lancet Oncol. 2019;20(12):1730-1739.
- de Wit R, de Bono J, Sternberg CN, et al. Cabazitaxel versus Abiraterone or Enzalutamide in Metastatic Prostate Cancer. N Eng J Med. 2019;381(26):2506-2518.
- Rubin MA, Maher CA, Chinnaiyan AM. Common gene rearrangements in prostate cancer. J Clin Oncol. 2011;29(27):3659-3668.
- Kunkel TA, Erie DA. DNA mismatch repair. Annu Rev Biochem. 2005;74:681-710.
- Pritchard C, Mateo J, Walsh M, De Sarkar N, Abida W, & Beltran H et al. Inherited DNA-Repair Gene Mutations in Men with Metastatic Prostate Cancer. N Eng J Med. 2016;375(5), 443-453.
- FDA Approves First PARP Inhibitor Rucaparib for Men with Metastatic Castration-Resistant Prostate Cancer (mCRPC) and a Deleterious BRCA Mutation. (2020). UroToday. Retrieved June 23, 2020, from https://www.urotoday.com/recent-abstracts/urologic-oncology/prostate-cancer/121465-fda-approves-first-parp-inhibitor-rucaparib-for-metastatic-castration-resistant-prostate-cancer-mcrpc.html
- Olaparib Approved in the US for HRR Gene-Mutated Metastatic Castration-Resistant Prostate Cancer. (2020). UroToday. Retrieved June 23, 2020, from https://www.urotoday.com/recent-abstracts/urologic-oncology/prostate-cancer/121612-lynparza-olaparib-approved-in-the-us-for-hrr-gene-mutated-metastatic-castration-resistant-prostate-cancer.html
- de Bono J, Mateo J, Fizazi K, Saad F, et al. Olaparib for Metastatic Castration-Resistant Prostate Cancer. N Eng J Med. 2020; 382 (22):2091-2102.
- Abida W, Bryce AH, Vogelzang N, et al. Preliminary Results From TRITON2: A Phase II Study of Rucaparib in Patients with mCRPC Associated with Homologous Recombination Repair Gene Alterations. Ann Oncol. 2018;29(Suppl 8):VIII271.
- Smith M, Saad F, Chowdhury S, Oudard S, Hadaschik B, & Graff J, et al. Apalutamide (APA) and overall survival (OS) in patients (pts) with nonmetastatic castration-resistant prostate cancer (nmCRPC): Updated results from the phase III SPARTAN study. Ann Oncol. 2019; 30(11):1813-1820.
- Yu EY, Massard C, Retz M, et al. Keynote-365 cohort a: Pembrolizumab (pembro) plus olaparib in docetaxel-pretreated patients (pts) with metastatic castrate-resistant prostate cancer (mCRPC). J Clin Oncol. 2019;37(Suppl 7):145.
- Clarke N, Wiechno P, Alekseev B, et al. Olaparib combined with abiraterone in patients with metastatic castration-resistant prostate cancer: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Oncol. 2018;19(7):975-986.
- Sweeney C, Gillessen-Sommer S, Rathkopf D, Matsubara N, Drake CG. IMbassador250: A phase III trial comparing atezolizumab with enzalutamide vs enzalutamide alone in patients with metastatic castration-resistant prostate cancer (mCRPC). AACR Annual Meeting. 2020;Abstr CT014.
- Agarwal N, Loriot Y, McGregor BA, Dreicer R, Dorff TB. Cabozantinib (C) in combination with atezolizumab (A) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC): Results of Cohort 6 of the COSMIC-021 Study. J Clin Oncol. 2020;38(Suppl 6):abstr 139.
- Kwon ED, Drake CG, Scher HI, et al. Ipilimumab versus placebo after radiotherapy in patients with metastatic castration-resistant prostate cancer that had progressed after docetaxel chemotherapy (CA184-043): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2014;15(7):700-712.
- Beer TM, Kwon ED, Drake CG, et al. Randomized, Double-Blind, Phase III Trial of Ipilimumab Versus Placebo in Asymptomatic or Minimally Symptomatic Patients With Metastatic Chemotherapy-Naive Castration-Resistant Prostate Cancer. J Clin Oncol. 2017;35(1):40-47.
- Hofman M, Violet J, Hicks R, Ferdinandus J, Thang S, Akhurst T et al. [ 177 Lu]-PSMA-617 radionuclide treatment in patients with metastatic castration-resistant prostate cancer (LuPSMA trial): a single-centre, single-arm, phase 2 study. Lancet Oncol. 2018;19(6):825-833.
- Seifert R, Kessel K, Schlack K, Weckesser M, Bogemann M, Rahbar K. Radioligand therapy using [(177)Lu]Lu-PSMA-617 in mCRPC: a pre-VISION single-center analysis. Eur J Nucl Med Mol Imaging. 2020. ePub ahead of print.
- Hofman M, Emmett L, Violet J, et al. TheraP: a randomized phase 2 trial of 177Lu-PSMA-617 theranostic treatment vs cabazitaxel in progressive metastatic castration-resistant prostate cancer (Clinical Trial Protocol ANZUP 1603). BJU International. 2019;124 (Suppl 1): 5-13
- Gravis G, Fizazi K, Joly F, et al. Androgen-deprivation therapy alone or with docetaxel in non-castrate metastatic prostate cancer (GETUG-AFU 15): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14(2):149-158.
- Tucci M, Bertaglia V, Vignani F, et al. Addition of Docetaxel to Androgen Deprivation Therapy for Patients with Hormone-sensitive Metastatic Prostate Cancer: A Systematic Review and Meta-analysis. Eur Urol. 2016;69(4):563-573.
- Kyriakopoulos CE, Chen YH, Carducci MA, et al. Chemohormonal Therapy in Metastatic Hormone-Sensitive Prostate Cancer: Long-Term Survival Analysis of the Randomized Phase III E3805 CHAARTED Trial. J Clin Oncol. 2018;36(11):1080-1087.
- James ND, de Bono JS, Spears MR, et al. Abiraterone for Prostate Cancer Not Previously Treated with Hormone Therapy. N Eng J Med. 2017;377(4):338-351.
- Fizazi K, Tran N, Fein L, et al. Abiraterone plus Prednisone in Metastatic, Castration-Sensitive Prostate Cancer. N Eng J Med. 2017;377(4):352-360.
- Armstrong AJ, Szmulewitz RZ, Petrylak DP, et al. ARCHES: A Randomized, Phase III Study of Androgen Deprivation Therapy With Enzalutamide or Placebo in Men With Metastatic Hormone-Sensitive Prostate Cancer. J Clin Oncol. 2019;37(32):2974-2986.
- Davis ID, Martin AJ, Stockler MR, et al. Enzalutamide with Standard First-Line Therapy in Metastatic Prostate Cancer. N Eng J Med. 2019;381(2):121-131.
- Chi KN, Agarwal N, Bjartell A, et al. Apalutamide for Metastatic, Castration-Sensitive Prostate Cancer. N Eng J Med. 2019;381(1):13-24.
- National Comprehensive Cancer Network. Prostate Cancer (Version 4.2019). https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed January 13, 2020.
- Wallis CJD, Klaassen Z, Bhindi B, et al. Comparison of Abiraterone Acetate and Docetaxel with Androgen Deprivation Therapy in High-risk and Metastatic Hormone-naive Prostate Cancer: A Systematic Review and Network Meta-analysis. Eur Urol. 2018;73(6):834-844.
- Sathianathen NJ, Koschel S, Thangasamy IA, et al. Indirect Comparisons of Efficacy between Combination Approaches in Metastatic Hormone-sensitive Prostate Cancer: A Systematic Review and Network Meta-analysis. Eur Urol. 2019;77(3):365-372
- Marchioni M, Di Nicola M, Primiceri G, et al. New Anti-Androgen Compounds Compared to Docetaxel in Metastatic Hormone Sensitive Prostate Cancer: Results from a Network Meta-Analysis. J Urol. 2019;203(4):751-759.
- Sternberg CN, Fizazi K, Saad F, et al. Enzalutamide and Survival in Nonmetastatic, Castration-Resistant Prostate Cancer. N Eng J Med. 2020;382(23):2197-2206.
- Hird AE, Magee DE, Bhindi B, et al. A Systematic Review and Network Meta-analysis of Novel Androgen Receptor Inhibitors in Non-metastatic Castration-resistant Prostate Cancer. Clin Genitourin Cancer. 2020;43(4):288-297.
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): a randomised controlled phase 3 trial. Lancet Oncol. 2018;392(10162):2353-2366.
- Boeve LMS, Hulshof M, Vis AN, et al. Effect on Survival of Androgen Deprivation Therapy Alone Compared to Androgen Deprivation Therapy Combined with Concurrent Radiation Therapy to the Prostate in Patients with Primary Bone Metastatic Prostate Cancer in a Prospective Randomised Clinical Trial: Data from the HORRAD Trial. Eur Urol. 2019;75(3):410-418.
From the Desk of the Editor: Volume 5, Issue 2
ARAMIS: Favorable Overall Survival and Safety Findings for Darolutamide in Nonmetastatic Castration-Resistant Prostate Cancer


