- Improving downstaging of disease
- Improving systemic disease control in the neoadjuvant period
- Consolidating local tumor control
- Improving systemic disease control in the adjuvant period
Dr. Arjun Balar presented “Pembrolizumab in combination with gemcitabine and concurrent hypofractionated radiation therapy as bladder sparing treatment for muscle-invasive bladder cancer: A multicenter phase 2 trial” and Dr. Xavier Garcia del Muro presented “Phase II trial of durvalumab plus tremelimumab with concurrent radiotherapy in patients with localized muscle-invasive bladder cancer treated with a selective bladder preservation approach: IMMUNOPRESERVE-SOGUG trial.” Despite these trials looking quite similar, Dr. Necchi notes that the pembrolizumab trial is using a radiosensitizing effect of checkpoint inhibition with a primary endpoint of clinical complete response, and the IMMUNOPRESERVE trial is using systemic tumor control of immunotherapy added to a putative abscopal effect of local radiotherapy, with a primary endpoint of bladder intact-disease free survival. Additional key differences in these two trials are as follows: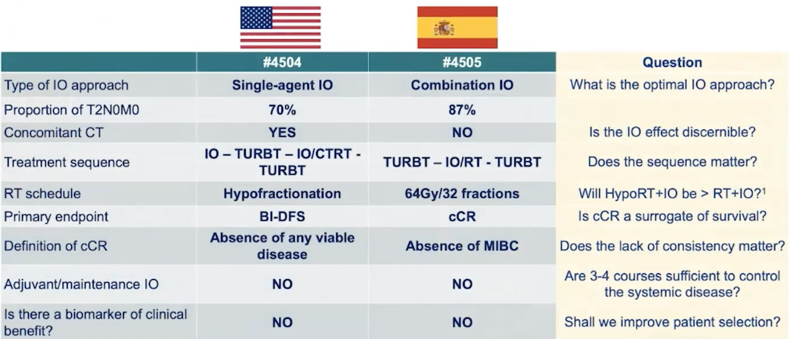
Dr. Necchi notes that we currently do not know if the therapeutic sequence matters, however, there are several trials ongoing assessing concomitant chemoradiation-immunotherapy, including the SWOG-1806 (testing atezolizumab) and KEYNOTE-992 (testing pembrolizumab). There is evidence for immunotherapy controlling local disease in the setting of short course of neoadjuvant immunotherapy prior to cystectomy, as has been evaluated in the NABUCCO, ABACUS, and PURE-01 studies. Presented at GU ASCO 2021 the RETAIN BLADDER study is a phase II trial of risk-enabled therapy after initiating neoadjuvant chemotherapy for bladder cancer. Following neoadjuvant chemotherapy, patients who had at least one mutation and no clinical evidence of disease by restaging TUR and imaging began a pre-defined active surveillance regime. The remaining patients who did not meet these criteria underwent bladder-directed therapy: intravesical therapy (< cT2 post- neoadjuvant chemotherapy), chemoradiation, or radical cystectomy:

There were 77 patients enrolled in the intention-to-treat population over 33 months at four academic centers. Among these patients 32 underwent radical cystectomy, 5 underwent chemoradiation and 7 underwent intravesical therapy, at some point during the trial. Overall, 55% of patients in the ITT cohort and 89% of patients in the active surveillance group had successful bladder preservation. In terms of overall survival, the majority of patients were alive and without metastasis at median follow-up between 18 and 20 months, in both the ITT and active surveillance populations.
The HCRN GU-257 trial was presented by Dr. Matt Galsky entitled “Phase 2 trial of gemcitabine, cisplatin, plus nivolumab with selective bladder sparing in patients with muscle-invasive bladder cancer: HCRN GU 16-257.” This trial had 31 patients with a clinical complete response, of which only one patient chose radical cystectomy and 30 patients opted for no cystectomy, but nivolumab for an additional 4 months. Dr. Necchi highlights that patient adherence to a therapeutic option that avoids surgery or radiation is high and is important for designing future clinical trials.
For these three trials, compared to the benchmark chemoradiation study1, Dr. Necchi provides the following scorecard based on short-term (secondary) outcomes: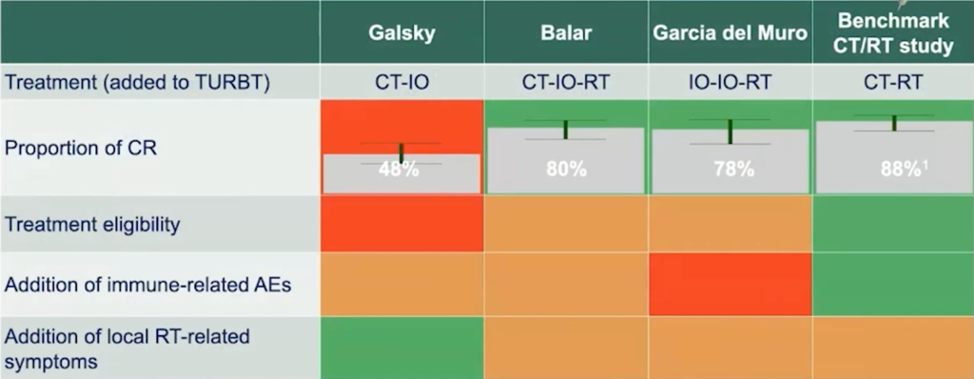
Furthermore, comparing the three trials for 12-month bladder intact-disease free survival, there is a slight advantage for Balar et al. (88%), however, the 78% reported by Galsky et al. spares the potential added morbidity by avoiding radiotherapy:
Moving forward, Dr. Necchi notes that it is important to consider tumor imaging, tumor biomarkers, and the utilization of new drugs. Multi-parametric MRI is a non-invasive way of diagnosing and assessing tumor response, which has been assessed in the BladderPath trial (n = 24)2 and the PURE-01 trial (n = 164)3. In BladderPath, 50 of 52 participants designated Likert 1-2 (probable NMIBC) from both pathways (TURBT or mpMRI) were confirmed as having NMIBC (96%). Ten of 11 cases diagnosed as NMIBC by mpMRI have been pathologically confirmed as NMIBC, and 10/15 cases diagnosed as MIBC by mpMRI have been treated as MIBC (5 participants underwent TURBT). Dr. Necchi highlighted that the PURE-01 study had an internal AUC of radiologic complete response for T0 of 0.76 (95% CI 0.68-0.83) and an external AUC of 0.74 (95% CI 0.66-0.82):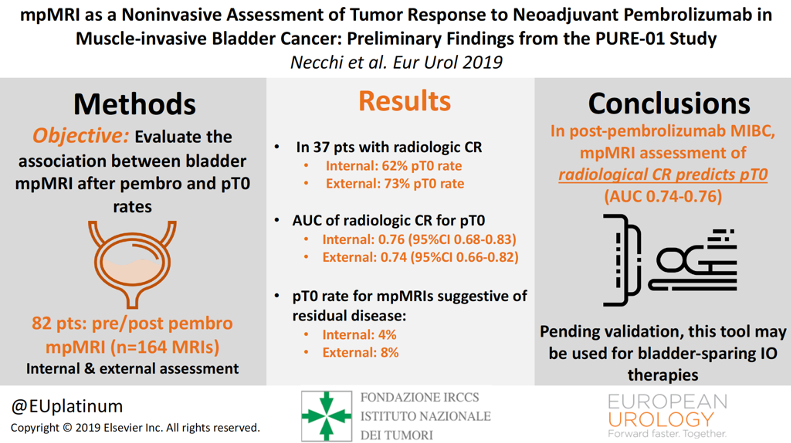
Moving forward, we need to keep incorporating new ‘players’ into rational therapeutic sequences. In a recently published study led by Dr. Necchi4, molecular subtyping of pre-and post-pembrolizumab samples revealed that only 36% of samples had a concordant subtype according to the consensus classifier. Unsupervised consensus clustering revealed three distinct post-pembrolizumab clusters (basal, luminal, and scar-like). A scar-like subtype was present in 50% of the post-pembrolizumab cases (n = 13) and expressed genes associated with wound healing/scarring. This subtype had higher luminal marker expression in the post-pembrolizumab setting compared to consensus clustering scar-like tumors from the other cohorts. Patients with the scar-like subtype showed a favorable prognosis after systemic therapy, but not in the radical cystectomy-only setting. As such, biomarkers will be important for potentially improving patient selection.
Dr. Necchi concluded with the following summary statements:
- The possibilities to improve or overcome the current therapeutic protocols by introducing immunotherapy will benefit from improvements in (i) harmonization of terminology and definitions of trial endpoints, (ii) longer follow-up time to confirm associations with outcomes, (iii) a substantial raise in the bar of therapeutic efficacy given that the benchmark chemoradiation therapy is hard to beat, and (iv) results from randomized trials to assess the magnitude of immunotherapy benefit
- Collaboration and teamwork benefit the patients at the highest level
Presented By: Andrea Necchi, MD, Fondazione IRCCS Istituto Nazionale dei Tumori, Milan, Italy
Written By: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md, at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021
References:
- Choudhury A, Swindell R, Logue JP, et al. Phase II study of conformal hypofractionated radiotherapy with concurrent gemcitabine in muscle-invasive bladder cancer. J Clin Oncol. 2011 Feb 20;29(6):733-738.
- Bryan RT, Liu W, Pirrie SJ, et al. Comparing an image-guided pathway with the standard pathway for staging muscle-invasive bladder cancer: Preliminary data from the BladderPath study. Eur Urol. 2021 Feb 27;S0302-2838(21)00141-x.
- Necchi A, Bandini M, Calareso G, et al. Multiparametric magnetic resonance imaging as a noninvasive assessment of tumor response to neoadjuvant pembrolizumab in muscle-invasive bladder cancer: Preliminary findings from the PURE-01 study. Eur Urol. 2020 May;77(5):636-643.
- Necchi A, de Jong JJ, Raggi D, et al. Molecular characterization of residual bladder cancer after neoadjuvant pembrolizumab. Eur Urol. 2021 Mar 27;S0302-2838(21)00212-8.


