This multicenter phase 2 trial included patients with cT2 – T4aN0M0 muscle-invasive bladder cancer who declined or were ineligible for cystectomy, ECOG performance status 0/1, eGFR > 30 cc/min, and no contraindications to pelvic radiotherapy or pembrolizumab. No perioperative chemotherapy for muscle-invasive bladder cancer was permitted. Patients received pembrolizumab 200 mg x 1 followed 2-3 weeks by maximal TURBT and then whole bladder radiotherapy (52 Gy/20 fractions; IMRT preferred) with twice-weekly gemcitabine 27 mg/m2 and pembrolizumab Q3 weeks x 3 treatments. 12 weeks after radiotherapy, CT/MR abdomen/pelvis, TUR of the tumor bed and cytology were performed to document response. Up to six patients were enrolled to a safety cohort followed by 48 patients in the efficacy cohort. The trial schema is as follows:
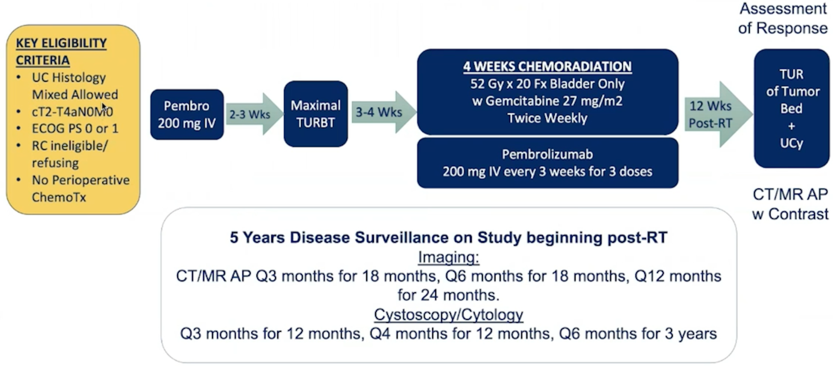
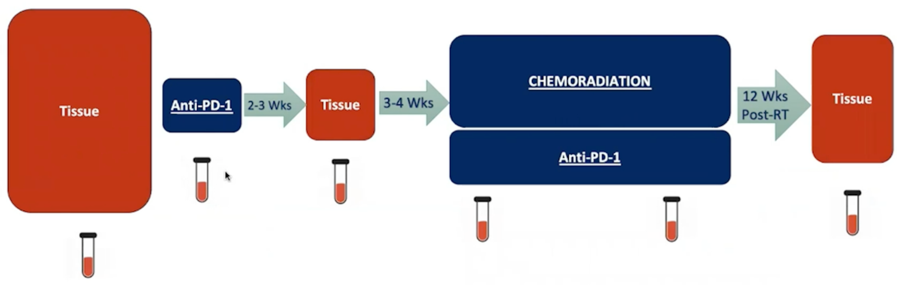
The primary endpoint is 2-year bladder-intact disease-free survival, defined as first of muscle-invasive bladder cancer or regional nodal recurrence, distant metastases, or death, assessed by serial cystoscopy/cytology and CT/MRI. The efficacy cohort had 85% power to detect a 20% absolute improvement in 2-yr bladder-intact disease-free survival rate over 60% historical rate.2 Key secondary endpoints were safety, 12 weeks complete response rate, metastases-free survival and overall survival. Tumor tissue was collected at study entry, maximal TURBT and post-treatment TUR of tumor bed with serial PBMCs for correlative analyses:
From May 2016 to October 2020, 54 patients (six in the safety cohort and 48 in the efficacy cohort) enrolled at 5 centers. The median age was 67 (range: 65-89) years for the safety cohort and 74 (range: 51-97) years for the efficacy cohort. The clinical stage was 70% cT2, 26% cT3, and 4% cT4. Overall, there were 39 (72%) patients that declined cystectomy. All six patients in the safety cohort and 42/48 (88%) patients in the efficacy cohort completed all study therapy. 1/48 (2%), 2/48 (4%), and 4/48 (8%) patients discontinued radiotherapy/gemcitabine, gemcitabine or pembrolizumab, respectively, most often due to toxicity.
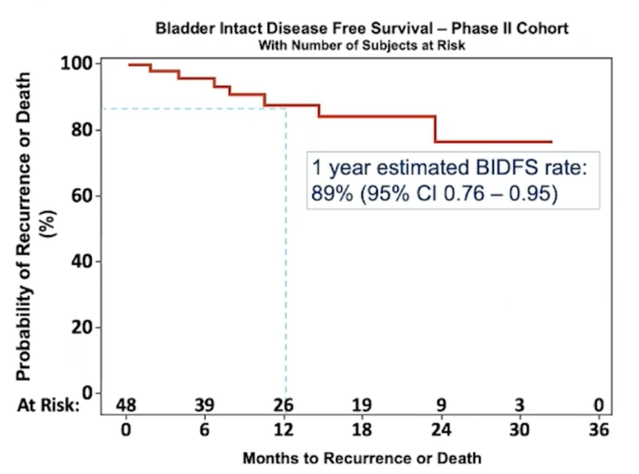
As of January 2021, with a median follow-up of 40.9 months (range: 38.6-50.8) in the safety cohort and 11.7 months (range: 0.6 – 32.2) in the efficacy cohort, there were no recurrences in the safety cohort, and 12/48 efficacy cohort patients had any recurrence (6 NMIBC, 0 muscle-invasive bladder cancer, 2 regional and 4 distant). The estimated 1 year bladder-intact disease-free survival rate in the efficacy cohort (n=48) is 88% (95% CI: 0.73-0.95):

The estimated 1 year bladder-intact disease-free survival rate in the overall cohort (n=54) is 89% (95% CI: 0.76-0.95):
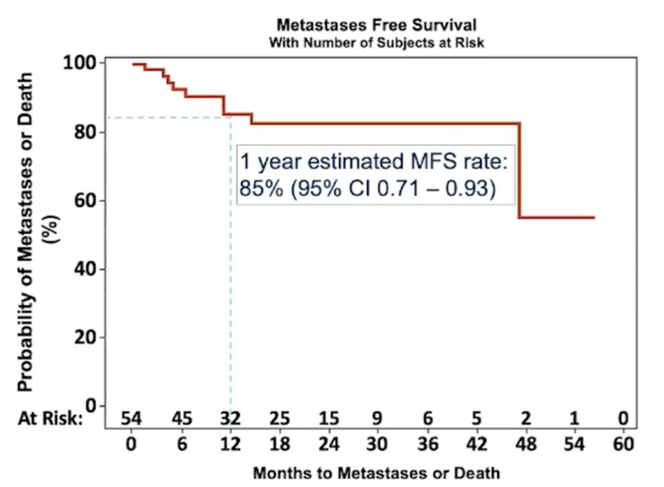
12 weeks complete response rate was 100% in safety cohort and 83% for the efficacy cohort (1 partial response, 3 no-response, 1 progression, 11 NE; two patients still on active study). The key secondary endpoint of 1-year metastasis-free survival rate in all patients was 85% (95% CI 0.71-0.93):
In the efficacy cohort, 35% of patients had a grade ≥3 treatment-emergent adverse events (grade 3 events included UTI 8%, diarrhea 4%, colitis 4%, bladder pain/obstruction 4%, neutropenia 2%, thrombocytopenia 2%). Notable pembrolizumab grade ≥3 treatment-emergent adverse events included 3 patients (6%) grade 3 GI toxicity and one patient grade 4 colonic perforation. One patient died due to fungemia, unrelated to study therapy.
Dr. Arjun Balar concluded his presentation of this phase 2 trial with the following take-home messages:
- Trimodality bladder preservation therapy is an effective non-surgical option for patients with muscle-invasive bladder cancer with curative intent
- Pembrolizumab added to hypofractionated radiotherapy and twice weekly gemcitabine was well-tolerated with promising efficacy in this early analysis: 88% rate of bladder intact disease-free survival at 1 year in the efficacy cohort is noteworthy, but longer follow-up is needed
- Pembrolizumab-related toxicity was consistent with prior monotherapy trials, including immune-related toxicities requiring steroids, however the majority were manageable
- Correlative studies on serially collected tumor tissue and blood are underway to interrogate the local and systemic impact of pembrolizumab added to chemoradiation on anti-tumor immunity
- Ongoing randomized studies including SWOG 1806 and KEYNOTE 992 will better define the role for immunotherapy added to trimodality bladder preservation
Clinical trial information: NCT02621151
Presented by: Arjun V. Balar, MD, Director of the Genitourinary Medical Oncology Program at NYU Langone’s Perlmutter Cancer Center, New York, NY
Co-Authors: Matthew I. Milowsky, Peter H. O'Donnell, Ajjai Shivaram Alva, Marisa Kollmeier, Tracy L Rose, Sean Pitroda, Samuel D. Kaffenberger, Jonathan E. Rosenberg, Kaitlyn Francese, Tsivia Hochman, Judith D Goldberg, Sarah Griglun, Dayna Leis, Gary D. Steinberg, James Wysock, Peter B. Schiff, Nicholas J. Sanfilippo, Samir Taneja, William C. Huang; University of North Carolina Department of Medicine, Division of Hematology/Oncology, Chapel Hill, NC; University of Chicago Comprehensive Cancer Center, Chicago, IL; University of Michigan Rogel Cancer Center, Ann Arbor, MI; Memorial Sloan Kettering Cancer Center, New York, NY; The University of North Carolina at Chapel Hill (UNC-CH) School of Medicine and UNC Lineberger Comprehensive Cancer Center, Chapel Hill, NC; University of Chicago, Chicago, IL; University of Michigan, Ann Arbor, MI; Genitourinary Medical Oncology Service, Division of Solid Tumor Oncology, Memorial Sloan Kettering Cancer Center, New York, NY; New York University School of Medicine, New York, NY; Department of Surgery, The University of Chicago Medicine, Chicago, IL; Department of Urology, New York University School of Medicine, New York, NY; Laura and Isaac Perlmutter Cancer Center, NYU Langone Health, New York, NY; Weill Cornell Medicine, New York, NY; NYU Langone Health, New York, NY; NYU Langone Medical Center, New York, NY
References:
- Kulkarni GS, Hermanns T, Wei Y, et al. Propensity score analysis of radical cystectomy versus bladder-sparing trimodal therapy in the setting of a multidisciplinary bladder cancer clinic. J Clin Oncol 2017;35(20):2299-2305.
- Mak RH, Hunt D, Shipley WU, et al. Long-term outcomes in patients with muscle-invasive bladder cancer after selected bladder-preserving combined-modality therapy: A pooled analysis of Radiation Therapy Oncology Group protocols 8802, 8903, 9506, 9706, 9906, and 0233. J Clin Oncol. 2014 Dec 1;32(34):3801-3909.


