

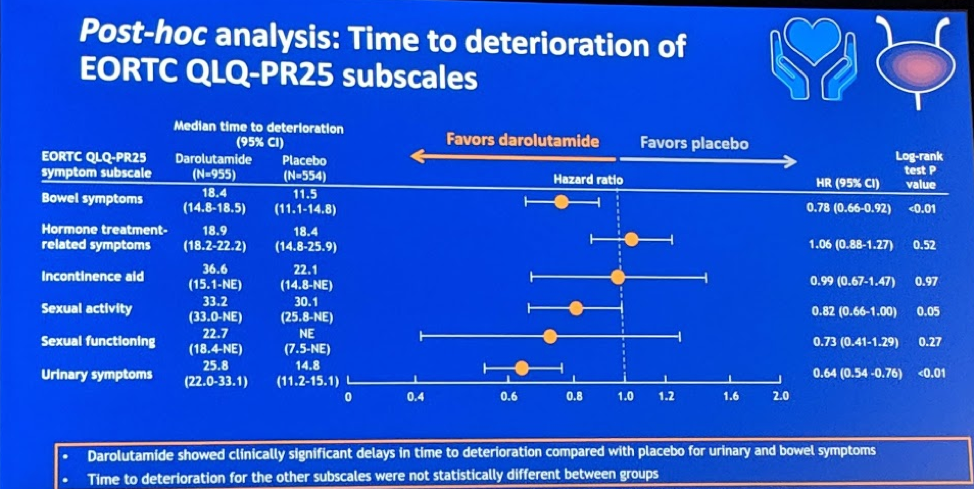
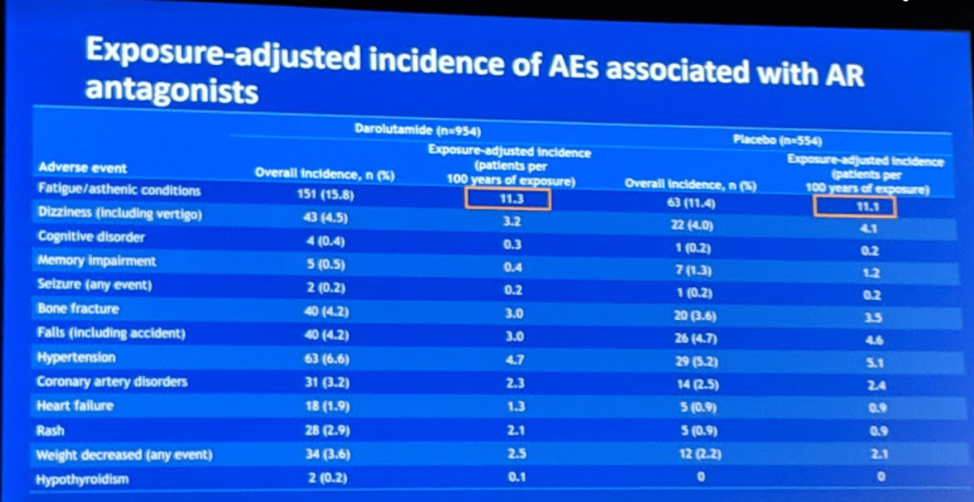
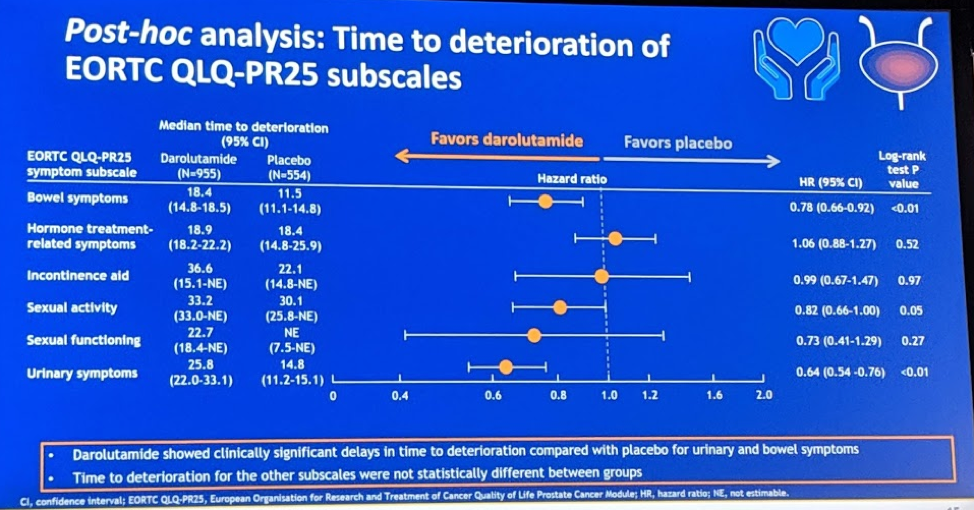
This abstract describes the pain progression and quality of life outcomes for 1509 patients who were randomly assigned to darolutamide or placebo in a 2:1 ratio. Darolutamide significantly delayed pain progression (40.3 vs 25.4 mo; HR 0.65; 95% CI 0.53–0.79; P < 0.001) and delayed urinary symptoms (25.8 vs 14.8 mo; HR 0.64; 95% CI 0.54–0.76; P < 0.01). Given the additional androgen blockade, hormone treatment-related symptoms were also analyzed and there was no difference in symptoms between patients on darolutamide or placebo (18.9 vs 18.4 mo; HR 1.06; 95% CI 0.88–1.27; P = 0.52). In terms of adverse events of interest, there was no significant difference in fatigue, hypertension, hot flushes, fractures, falls, cognitive disorders, or seizures.
Written by: Jason Zhu, MD, Fellow, Division of Hematology and Oncology, Duke University Twitter: @TheRealJasonZhu at the 2019 ASCO Annual Meeting #ASCO19, May 31-June 4, 2019, Chicago, IL USA
References:
- Fizazi K, Smith MR, Tombal B Clinical Development of Darolutamide: A Novel Androgen Receptor Antagonist for the Treatment of Prostate Cancer. Clin Genitourin Cancer. 2018 Oct;16(5):332-340. doi: 10.1016/j.clgc.2018.07.017. Epub 2018 Jul 24,
- Fizazi K, Shore N, Tammela TL, et al. Darolutamide in Nonmetastatic, castration-resistant prostate cancer. New England Journal of Medicine 2019.
- Hussain M, Fizazi K, Saad F, et al. PROSPER: A phase 3, randomized, double-blind, placebo (PBO)-controlled study of enzalutamide (ENZA) in men with nonmetastatic castration-resistant prostate cancer (M0 CRPC). American Society of Clinical Oncology; 2018.
- Small EJ, Saad F, Chowdhury S, et al. SPARTAN, a phase 3 double-blind, randomized study of apalutamide (APA) versus placebo (PBO) in patients (pts) with nonmetastatic castration-resistant prostate cancer (nmCRPC). American Society of Clinical Oncology; 2018.


