Two patient cohorts were studied to evaluate effects of AV002, emulsified microdose of vasopressin, on the number of nocturic episodes, length of FUSP, and percentage of one or less nocturic voids per night. Impact of Nighttime Urination (INU) questionnaire was administered to capture patient perception on the quality of life improvement. Adverse events assessment included analysis of cases of hyponatremia. Moderate hyponatremia was defined as 126-129 mmol/L, and severe hyponatremia constituted serum sodium level of ≤125 mmol/L.
Researchers analyzed two phase 3 studies conducted to date in patients ≥65 and ≥75 years of age. Nocturia was defined as 2 or more nighttime voids per night for ≥ 6 months. Subjects with BPH and OAB were allowed to participate in the trial. Participants were randomized into 3 groups: intranasal AV002 1.66mcg, AV002 0.83mcg, or placebo. Study sample characteristics included in Figure 1:

Figure 1
Results showed that medication was effective in reducing nocturic voids in both groups (Figure 2).
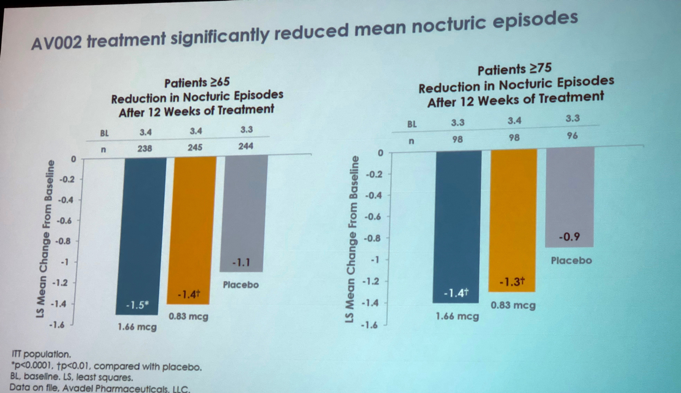
Figure 2
Both treatment groups also exhibited significant improvement in FUSP to up to 4 hours compared to the placebo arm (Figure 3).

Figure 3
In addition, the study demonstrated an increase in a number of nights with one or less nocturic voids in 0.83mcg and 1.66mcg groups compared to the placebo group. Subjects didn’t have any severe adverse events but reported mild nasal discomfort and irritation. Although hyponatremia was reported in some research subjects, incidence was low for both doses. According to the presenter, severe hyponatremia was documented in subjects who took the highest dose of medication (1.66 mcg), but it could be linked to other contributing factors.
AV002 could be recommended for the elderly population with nocturia as a safe and reliable treatment option. There were no reports of severe adverse events related to the study drug in subjects who took the lowest dose of medication (0.83 mcg). This dose can be ideal therapy options for the studied cohort.
Presented by: Benjamin M. Brucker, MD, New York University Langone Health, New York, NY
Co-authors: Kathleen Kobashi, Leo Francis, Alex Yang, Diane K Newman.
Written by: Hanna Stambakio, BS, Clinical Research Coordinator, Division of Urology, University of Pennsylvania, @PennUrology at the 2018 ICS International Continence Society Meeting - August 28 - 31, 2018 – Philadelphia, PA USA
Read More from the Presenter, Benjamin Brucker, MD on UroToday Lower Urinary Tract Conditions Center of Excellence


