(UroToday.com) Dr. Elisabeth Heath provided a discussion following presentations from Dr. Smith and Dr. Merseberger on the ARASENS and PRESIDE trials, respectively In an oral abstract presentation on the first day of the American Society for Clinical Oncology (ASCO) Genitourinary Cancer Symposium 2022.
Dr. Heath began by highlighting the many and rapid changes that have changed in the treatment landscape for metastatic hormone-sensitive prostate cancer (mHSPC) over the past seven years, with androgen deprivation therapy (ADT) treatment intensification beginning with docetaxel in 2015 and subsequently including a number of monotherapy androgen receptor-axis-targeted therapies (ARATs) including abiraterone, enzalutamide, and apalutamide over the following 5 years. In the past year, we have seen the introduction of triplet therapy. As of ASCO 2021 and subsequently, at ESMO 2021, the PEACE-1 trial demonstrated the role of docetaxel and abiraterone in addition to ADT.

At GU-ASCO 2022, Dr. Smith presented results of the ARASENS trial which demonstrated a survival benefit to the combination of docetaxel and darolutamide in addition to ADT, compared to docetaxel and ADT.
Dr. Heath then provides a comparison of the survival benefit associated with treatment intensification for those trials that used a control arm of ADT alone. While there are clearly difference between the trials in terms of inclusion criteria and subsequent therapy, there is a relatively consistent magnitude of benefit to these treatment approaches.

Comparing the available data from PEACE-1 and ARASENS, docetaxel and abiraterone demonstrated a 25% relative improvement in overall survival while darolutamide and docetaxel demonstrated a 32.5% improvement in survival, each compared to docetaxel.
Dr. Heath then focused on the design of ARASENS, emphasizing that this trial included patients with mHSPC who were candidates for both ADT and docetaxel. In addition to ongoing ADT and docetaxel (in keeping with the CHAARTED defined standard of care), patients were randomized to darolutamide or placebo and were followed for the primary endpoint of overall survival.

In addition to demonstrated improvements in overall survival, Dr. Heath highlighted that the combination of darolutamide and docetaxel with ADT demonstrated improvements in secondary endpoints including time to castration resistant prostate cancer (CRPC), time to pain progression, symptomatic skeletal event free survival, time to first symptomatic skeletal event, and time to initiation of subsequent systemic antineoplastic therapy. However, there was no difference in the time to worsening of disease-related physical symptoms (HR 1.04, 95% CI 0.89-1.22), thus statistical comparison of time to initiation of opioid use for more than 7 consecutive days was not performed.
When examining treatment-emergent adverse events, there were common in both arms, including any events in 99.5% of patients in the darolutamide and docetaxel arm and 98.9% of patients in the docetaxel arm. Serious adverse events were somewhat higher in the combination arm as well (44.8% versus 42.3%). However, rates of AEs leading to permanent discontinuation were fairly comparable.
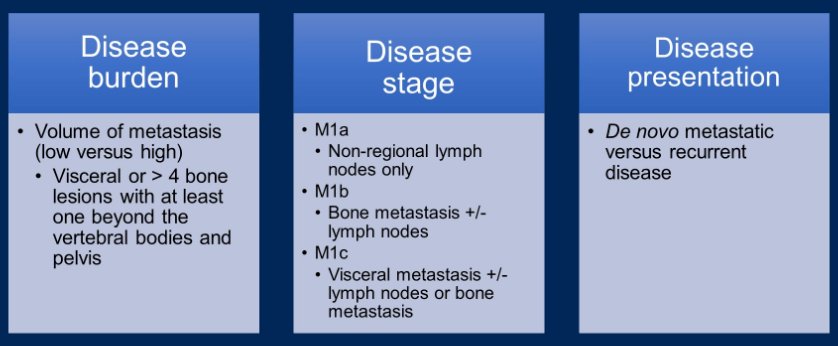
Considering the characteristics of patients included in the ARASENS trial, Dr. Heath emphasized that just under 80% of included patients had M1b disease, approximately 3% had M1a disease and 17-18% had M1c disease. Thus, moving forward, she emphasized the importance of a number of clinical consideration in treatment decision making including disease burden (the volume of metastasis, per CHAARTED criteria), disease stage, and disease presentation (de novo versus recurrent metastatic disease). Dr. Heath emphasized that de novo disease has a different biology, and worse prognosis, than recurrent metastatic disease.
Further, she emphasized considerations regarding molecular data (such as HRR gene mutations, and others), the role of local therapy, and the influence of prior treatments. In terms of take home messages, Dr. Heath emphasized that a triplet regime comprising ADT, docetaxel, and darolutamide is a new standard of care in mHSPC. She further emphasized that this triplet approach should be emphasized in patients with M1b and M1c disease ho are fit for a combination approach.
However, while there is great promise in triplet therapy, Dr. Heath emphasized that current uptake of doublet ADT treatment intensification is has been poor to date.

Thus, she emphasized the importance that more education, more communication, and more awareness is needed to ensure that our patients are receiving treatment with proven survival benefit. In the context, she cited the treatment landscape for mCRPC in which treatment intensification has been better adopted.

Transitioning to Dr. Merseburger’s presentation of the PRESIDE trial, Dr. Heath emphasized that this addresses a really clinical relevant question. Dr. Health emphasized that, based on the data from PREVAIL, enzalutamide is a commonly employed first-line treatment in mCRPC. This, therefore, forms the basis of period 1 of the PRESIDE trial. However, most patients will progress while on enzalutamide therapy. For eligible patients, docetaxel chemotherapy is the standard second-line treatment in mCRPC.

Dr. Heath emphasized that, while overall survival is commonly considered as the gold standard outcome, progression-free survival provides important information in this context. Summarizing these data, she emphasized that for patients with mCRPC who are progressing on enzalutamide, continuing enzalutamide while initiating docetaxel may provide an improvement in progression-free survival. She emphasized that a number of patient characteristics may particularly support this approach including an initial good response to enzalutamide, an ongoing clinical benefit with enzalutamide, and a fitness for combination therapy.
Summarizing both of these talks, Dr. Heath emphasized that there is considerable uncertainty in both mHSPC and mCRPC. We need to engage and encourage multidisciplinary team works to improve treatment strategies and consider disease burden and other factors to optimize treatment selection.
Presented by: Elisabeth I. Heath, MD, FACP, Karmanos Cancer Institute, Wayne State University School of Medicine
Written by: Christopher J.D. Wallis, University of Toronto, Twitter: @WallisCJD during the 2022 American Society of Clinical Oncology Genitourinary (ASCO GU) Cancers Symposium, Thursday Feb 17 – Saturday Feb 19, 2022


