When he addresses each of the trials, he asks the following question to determine if it should change clinical practice:
- 1) Is the condition one that requires treatment?
- 2) Is there a meaningful endpoint?
- 3) Does the agent have acceptable toxicity?
- 4) Does it compromise the efficacy of subsequent therapies?
- 5) Ideally, it must also be cost-effective
- Is the condition one that requires treatment?
- But, data by Smith et al. (JCO 2013), did demonstrate that men with cM0 PCa and PSADT <=6 months had significantly worse outcomes than men with PSA DT > 6 months
- So, men with cM0 PCa with PSA DT <= 6 months likely still warrant treatment.
- Meaningful endpoint? The primary endpoint was MFS, which it met. However, there is debate as to whether this translated to OS benefit. It was sufficient enough for the FDA to approve it based on MFS survival benefit alone. - Possible meaningful benefit
- It does have acceptable toxcicity
- It is uncertain whether or not it affects subsequent therapy effectiveness. No data for analysis.
- Cost-effectiveness – Unfortunately, as there is no price yet or OS data yet for darolutamide, there is no way to calculate ICER or cost-effectiveness.
LATITUDE
- Is the condition one that requires treatment?
- Meaningful endpoint? In this final analysis, they provide updated OS data. This is a meaningful endpoint. Compared to the interim results previously provided, the OS data has matured, but remains very significant. There is a 36% risk reduction (HR 0.66, p < 0.0001).
- Toxicity – In their QOL and safety analysis, they note that patients on abiraterone vs. placebo have much higher QOL scores throughout the course of the study. The adverse event profile was also appropriate for abiraterone.
- Does it affect subsequent treatment?
- Cost-effectiveness: This is the only criteria that it perhaps does not meet. The cost of abiraterone is ~$10K/month. As mentioned earlier in the day by Ronald Chen, the incremental benefit of abiraterone in the United States exceeds the $100K/QALY for cost-effective therapy – but if does become generic, it may be considered cost-effective.
- In Australia, it is approved but not reimbursed
In the United States, the patent overturned in October 2018. The company has appealed in Jan 2019. In Europe, the exclusivity contract runs through 2022. So, it may be a while before it becomes generic and cost-effective. In conclusion, he notes that it SHOULD change practice, but only IF available and affordable. Based on subgroup analysis, the greatest benefit may be for low-volume disease – because for high-volume disease, docetaxel may be more cost-effective.
ARCHES
- Is the condition one that requires treatment?
- Meaningful endpoint? At this time, the primary endpoint in this first analysis is recurrence progression-free survival (rPFS), though early overall survival (OS) data was also reported.
- The OS data is immature, so an early signal may not be reflective of final results
However, rPFS was significantly improved with enzalutamide use. This benefit extended across subgroups, including low/high volume disease and in patients with prior docetaxel use.
In the following chart, he compares the trials in this disease space:
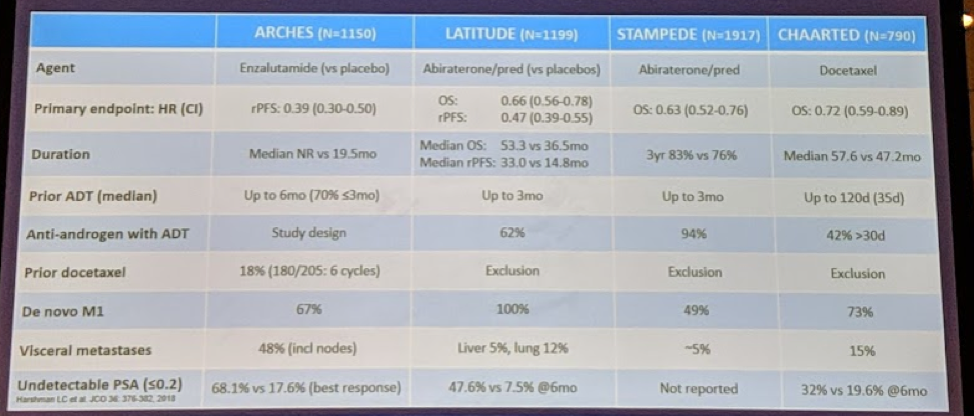
So, it is a questionable endpoint but may become reliable with more mature data.
- Toxicity – acceptable toxicity levels. Grade 3/4 AE were as expected. Mean Fact-P scores were no differences between treatment and placebo arms.
- Does it affect subsequent treatment efficacy?
- Is it cost-effective?
Presented by: Ian D. Davis, MBBS, Ph.D., FRACP, FAChPM, Monash University Eastern Health Clinical School
Written by: Thenappan Chandrasekar, MD (Clinical Instructor, Thomas Jefferson University) (twitter: @tchandra_uromd, @JEFFUrology) at the 2019 American Society of Clinical Oncology Genitourinary Cancers Symposium, (ASCO GU) #GU19, February 14-16, 2019 - San Francisco, CA
Further Related Content:
Read: Darolutamide Receives FDA Approval for the Treatment of Non-metastatic Castration-resistant Prostate Cancer


