Background: Sipuleucel-T is an autologous cellular immunotherapy approved for asymptomatic or minimally symptomatic metastatic castrate-resistant prostate cancer.
In the IMPACT trial, sipuleucel-T showed a 22.5% reduction in risk of death vs the control group (hazard ratio [HR]=0.775 [95% CI 0.614, 0.979]; P=0.032). A pre-specified subgroup analysis for baseline prognostic variables showed homogeneous treatment effects consistently favoring sipuleucel-T. In patients (pts) with baseline PSA below vs above the median, there was a trend toward greater treatment effect (HR=0.685 vs. 0.865). In this exploratory analysis, we further sub-divide baseline PSA into quartiles to evaluate potential treatment effect patterns.
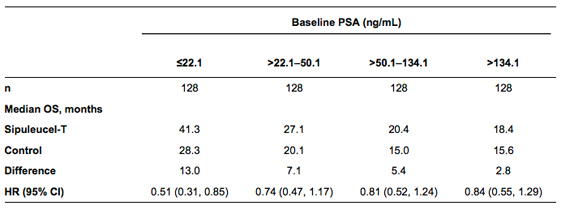
Methods: The analysis included all randomized pts from IMPACT (n=512). Pts were categorized by baseline PSA quartile (Table), ECOG PS and by median for other baseline prognostic variables (i.e., LDH, PAP, ALP in bone-only disease, and Hgb). Median OS and HR were estimated using Kaplan-Meier and Cox models, respectively.
Results: Increasing baseline PSA quartile was associated with markers of advanced disease. HRs suggest a consistent treatment effect in all subsets, although there is inadequate power to show significant results within each quartile. There was a trend toward an increased magnitude of treatment benefit in pts with a lower baseline PSA (Table). Results for other baseline prognostic variables also suggest a trend toward greater benefit in subjects with better prognostic features. However, results for baseline Hgb indicated an opposite trend.
Conclusions: Although not adequately powered for significance, the results of this analysis support a consistent OS benefit with sipuleucel-T across PSA quartiles. The greater magnitude of benefit in pts with lower baseline PSA suggests that pts with less advanced disease may benefit more from treatment with sipuleucel-T. Baseline PSA (ng/mL)
Written by:
Gerald Chodak, Paul F. Schellhammer, James Boyd Whitmore, Robert Brownell Sims, Philip W. Kantoff Are you the author?
Weiss Memorial Hospital, Chicago, IL; Eastern Virginia Medical School/Urology of Virginia, Norfolk, VA; Dendreon Corporation, Seattle, WA; Dana-Farber Cancer Institute, Boston, MA
Reference: J Clin Oncol 30, 2012 (suppl; abstr 4648)
UroToday.com Prostate Cancer Section



