The symposium was a three-day event sponsored by the Focal Therapy Society and the Endourological Society led by Dr. Thomas Polascik and Dr. Art Rastinehad. A distinguished faculty of 52 clinicians and researchers from around the globe discussed the latest developments in both therapy and imaging. The program included a variety of hands-on MRI and MR-targeted biopsy workshops, presentations and posters. While there has been an ongoing debate in the urological community for over a decade as to if focal therapy is feasible, symposium attendees discussed which focal therapies they are already or soon or may use in their clinical practice. The symposium’s tone was consistently positive and collaborative.
Here are a dozen highlights and takeaways:
1). There is a tremendous variety of focal therapies including cryotherapy, HIFU, MR-guided laser, VTP/TOOKAD, and numerous others. Presentations addressed the status of many trials around the world.
2). The emphasis on international contribution was stimulating and included outstanding speakers from outside of the U.S.: Dr. Laurence Klotz (Canada), Dr. Mark Emberton (UK), Dr. Jean de la Rosette (Turkey), Dr. Osamu Ukimura (Japan), Dr. Kae Jack Tay (Singapore), Dr. Rafael Sanchez Salas (France), Dr. Arnauld Villers (France), and others.
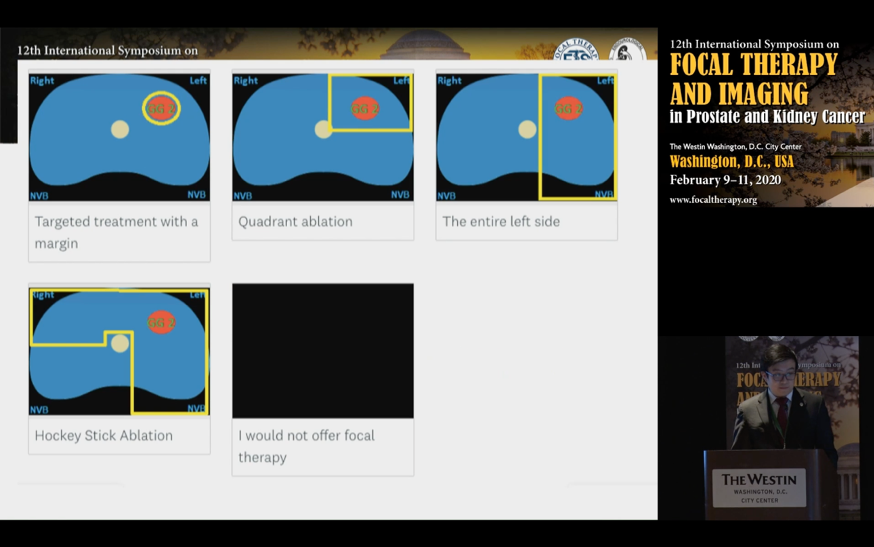
3). The definition of focal varies: “true” focal of specific lesions (plus margins), quadrant, hemi-gland, and irregular “hockey stick” patterns.
4). Presentations by Dr. Peter Carroll, Dr. Timothy McClure, Dr. William Linehan, and others explored the current and future use of various genetics biomarkers for renal and prostate cancer. Genetics will increasingly be used in combination with other biomarkers, MRI, and AI to guide clinicians as to treatment plans.
1). There is a tremendous variety of focal therapies including cryotherapy, HIFU, MR-guided laser, VTP/TOOKAD, and numerous others. Presentations addressed the status of many trials around the world.
2). The emphasis on international contribution was stimulating and included outstanding speakers from outside of the U.S.: Dr. Laurence Klotz (Canada), Dr. Mark Emberton (UK), Dr. Jean de la Rosette (Turkey), Dr. Osamu Ukimura (Japan), Dr. Kae Jack Tay (Singapore), Dr. Rafael Sanchez Salas (France), Dr. Arnauld Villers (France), and others.
3). The definition of focal varies: “true” focal of specific lesions (plus margins), quadrant, hemi-gland, and irregular “hockey stick” patterns.

4). Presentations by Dr. Peter Carroll, Dr. Timothy McClure, Dr. William Linehan, and others explored the current and future use of various genetics biomarkers for renal and prostate cancer. Genetics will increasingly be used in combination with other biomarkers, MRI, and AI to guide clinicians as to treatment plans.
5). Dr. John Fellers’ two presentations, "Phase II outpatient MR-guided laser focal therapy: 10-year interim results" and "'Trojan Horse' model for prostate cancer treatment using stromal vascular fraction (SVF) and oncolytic virus", were highlights of the symposium. In the future, combining focal therapy with novel immunotherapy may provide optimal outcomes.
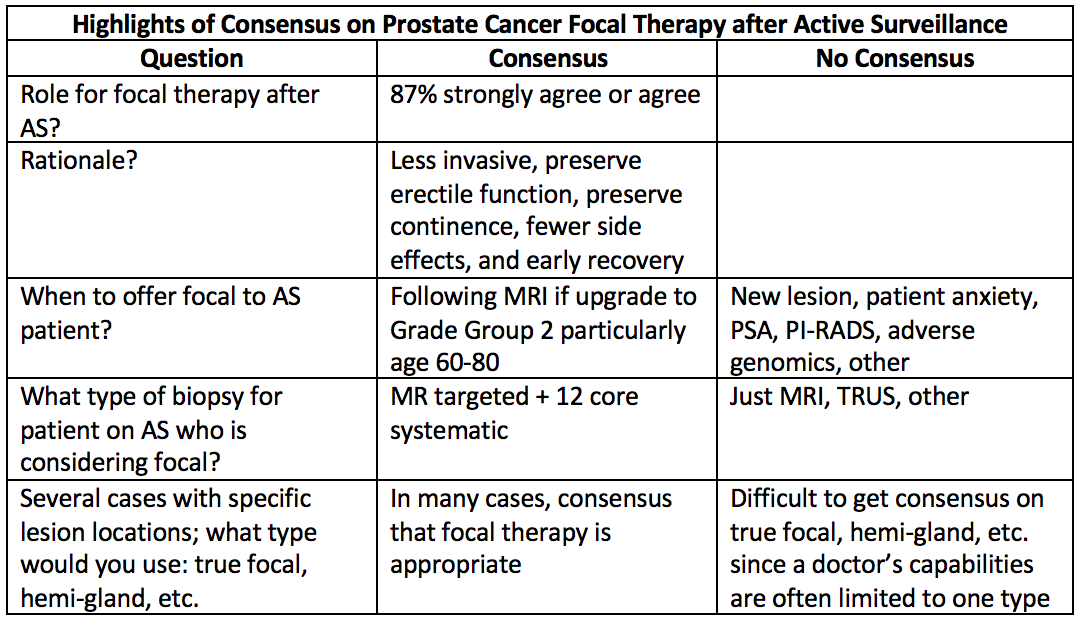
6). “Utilization of Focal Therapy for Patients Coming off Active Surveillance” was based on a multi-phase Delphi process. Led by Dr. Polascik, the panel consisted primarily of urologic oncologists from academia in larger cities. Attendees currently offer various whole gland and focal therapies: 79% laparoscopic/radical prostatectomy; 45% external beam radiation; 45% cryoablation; 43% brachytherapy; 41% HIFU; 34% open prostatectomy; 29% laser ablation; and <15% other modalities. The mean number of focal therapies performed in a year by each expert was 20. On each question, the consensus is defined as > 80% agreement. Note that the consensus is prevailing practice/opinion of experienced clinicians, and it is not a substitute for high-level evidence. See the following table for highlights.
7). “Is AS after FT the same as de novo AS?”: Dr. Klotz summarized his long-term research related to active surveillance. He emphasized the importance of periodic high-quality MRIs and biopsies. An important finding is that the average progression is 1-2% per year. Some believe that the progression is much higher; however, this is attributable primarily to a misattribution of Gleason grade, particularly from TRUS biopsies.

Later this year, Dr. Klotz plans to release a study of a liquid biomarker that appears to be extremely accurate at detecting Clinically Significant cancer.
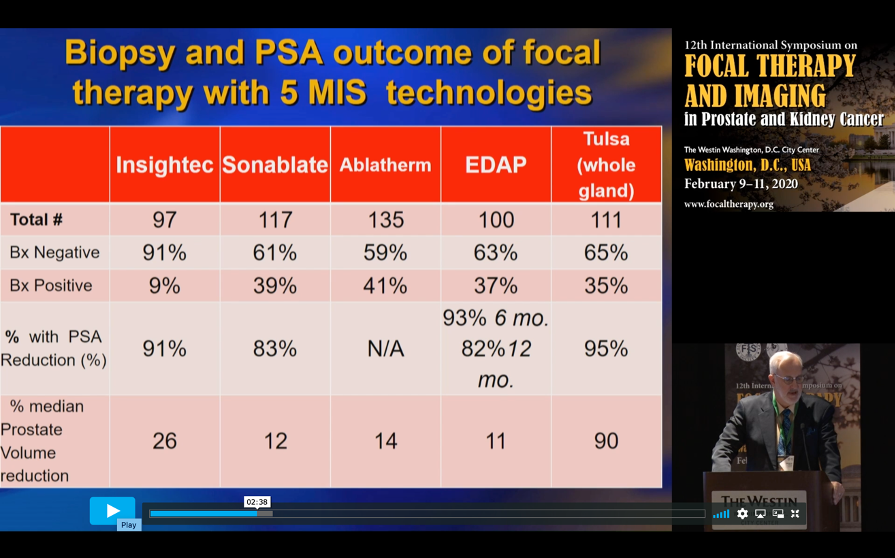
He also showed highlights from four suppliers’ focal high-intensity focused ultrasound (HIFU) studies and the whole-gland TULSA® trial (TACT):
8). There were a number of biopsy sessions including “A Negative Fusion Biopsy. What Did I Miss and What Is the Next Step?” Dr. Pinto explained: 1) the need for high-quality mpMRI imaging with an optimal magnet, coil, radiologist, and dedicated (prostate expert) technician; 2) the importance of high-quality MR-targeted fusion biopsy with optimal co-registration, needle insertion on a plane to avoid deflection, etc.; 3) inflammation can mimic cancer, and 4) compliance with and limitations of Pi-RAD v2.
At a separate session, Dr. Bradford Wood, an Interventional Radiologist at the NIH Clinical Center, addressed a variety of topics related to renal cell carcinoma ablation including fusion and “cool tools”. Other speakers including Dr. David Crawford, Dr. Michael Gorin, and Dr. Art Rastinehad who updated the audience at different sessions on the merits of transperineal versus transrectal biopsies for staging, planning, and treatments.
9). “Proposed New End-Point for Localized, Low-Risk Prostate Cancer Trials: Progression-Free Survival and Radical-Treatment-Free Survival”: Dr. Inderbir Gill (who is an unpaid advisor to Steba Biotech) gave a historical perspective on FDA approvals for low-risk prostate cancer. Following are his notes:

Dr. Gill stated that focal treatments must be “approved with a specific indication for prostate cancer. Until that specific indication shows up, the payers are not going to be interested in reimbursing it.” An FDA Public workshop on July 11, 2018 was held to discuss endpoints for localized low-risk disease. Dr. Gill continues, “The FDA and the urology community agree that overall survival, cancer-specific survival, and metastatic-free survival are not practical endpoints for clinical studies (Weinstock et al J Urology 2019).” Focal treatments’ future endpoints are not clear but may be based on avoiding disease progression (MRI and MR-targeted biopsy confirmed), patient-reported outcomes of side effects, and some measurement that delayed whole-gland treatment did not cause harm.

10). In his presentation titled “Are We Likely to Acquire a New Procedure Code for Fusion Biopsy?”, Dr. Norman Smith gave an excellent update on the arcane topic of fusion biopsy. He explained that after FDA approval of any procedure, the AMA may consider granting a new procedure code. However, this triggers a review of related codes which can be complex and is a “zero-sum game”. So, if there is a new fusion biopsy code, how will other biopsy codes be reimbursed? If a fusion biopsy gets a somewhat higher than average reimbursement, then TRUS biopsies could get a reduction from the current level. Dr. Smith then discussed the procedure code for MRI-targeted guidance. Is the current MRI guidance code just applicable to in-bore guidance, or will carriers also allow it for MR fusion guidance?
11). Presenting on “Incorporating Patient Preference Perspectives for Bovel PCa Therapies”, Charles Viviano, MD, PhD, FDA introduced a new FDA initiative to understand patients’ preferences. While IRB trials are needed, whole-gland trials’ endpoints may not be appropriate or feasible for focal therapies. So, focal therapy trials should encompass different endpoints, follow-up, and patient-reported outcome measurements.

Summary
The 12th Symposium was a great success. Attendees from around the world collaborated with colleagues to explore clinical trials, consensus statements, and “tips & tricks”. From discussions that I had with attendees, this symposium may be highly impactful on behavior. Researchers have a better understanding of new developments in trials’ results and future design. Clinicians practiced skills in workshops and are increasingly comfortable with integrating focal imaging and therapies into their clinical practice. So, many have clearly moved past the debate as to if focal makes sense and are exploring which tools should be used in specific situations. The Focal Therapy Society’s Board of Directors closed proceedings with an invitation to attend the 2021 Symposium in Turkey.
Written by: John Fortin, Retired Healthcare Actuary, Fellow in the Society of Actuaries, patient-advocate and prostate cancer survivor
Related Content:
Watch: Focal Therapy in Prostate Cancer - Art Rastinehad
Watch: The Evolution of Care: Focal Therapy for Prostate Cancer: Tom Polascik
Watch: Outpatient Trans-Rectal MR-Guided Laser Focal Therapy Phase II Clinical Trial: 10-Year Interim Results - John Feller
Related Content:
Watch: Focal Therapy in Prostate Cancer - Art Rastinehad
Watch: The Evolution of Care: Focal Therapy for Prostate Cancer: Tom Polascik
Watch: Outpatient Trans-Rectal MR-Guided Laser Focal Therapy Phase II Clinical Trial: 10-Year Interim Results - John Feller


