Secondary to the growing number of men living with prostate cancer, over the last decade or so there has been growing research and clinical interest in prostate cancer survivorship. This Center of Excellence article will review both the physical and psychological side effects of a prostate cancer diagnosis and treatment, highlight several tools for screening for psychological side effects amongst prostate cancer survivors, and discuss necessary steps and components for a multidisciplinary prostate cancer survivorship program.
Physical Side Effects
Urinary Dysfunction
Urinary dysfunction is a side effect of both surgical and radiation therapy for local treatment of prostate cancer. Surgical side effects include typically a period of urinary incontinence (ie. mixed incontinence) for several months post-operatively, followed by a degree of stress urinary incontinence that may persists for months or even years. Radiation therapy induced urinary dysfunction typically manifests as bladder irritability/over activity either during treatment or shortly thereafter. Longer term urinary dysfunction issues after radiation therapy may include urethral strictures necessitating periodic interventions and/or catheterization and stress urinary incontinence.
The ProtecT trial has arguably the best follow-up and comparison data for functional outcomes among men treated for localized prostate cancer. ProtecT randomized 1,643 men from 1999 to 2009 to undergoing either active monitoring (n=545), surgery (n=553), or radiotherapy (n=545), finding that at a median of 10 years of follow-up, prostate cancer specific mortality was low irrespective of treatment.2 As part of this trial, patient reported outcomes were collected and have now become one of the benchmarks for counseling patients with regards to long-term side effects of treatment for localized prostate cancer treatment.3 Questionnaires were completed at the time of diagnosis, at 6 and 12 months after randomization, and annually thereafter. Patients completed validated measures that assessed urinary, bowel, and sexual function and specific effects on quality of life, anxiety, and depression, and general health. Regarding urinary dysfunction, radical prostatectomy had the greatest negative effect on urinary continence, and although there was some recovery over time, these patients remained worse throughout follow-up compared to patients undergoing active monitoring or radiation therapy. Interestingly, radiation therapy had little effect on urinary incontinence, and there was a gradual decrease in urinary function over time for the men undergoing active monitoring. Urinary voiding and nocturia were worse in the radiotherapy group at 6 months but then mostly recovered and were similar to the other groups after 12 months:

Urinary incontinence has been cited as being the most important factor for decision regret among receiving local therapy for prostate cancer and may be incompletely explained/discussed with ~80% of patients prior to undergoing treatment.4
Sexual Dysfunction
Similar to urinary dysfunction, sexual dysfunction is a common side effect of localized therapy for prostate cancer. Patients undergoing radical prostatectomy will suffer a degree of sexual dysfunction in the immediate post-operative period with a degree of recovering over 12-24 months after surgery. Many studies have been published assessing predictors of post-operative recovery of sexual function, commonly highlighting younger age and adequate function pre-operatively as predictors of post-operative recovery. Men undergoing radiation therapy, similar to urinary dysfunction, will not notice an immediate effect on sexual function during the treatment phase, but generally suffer sexual dysfunction in the years post-radiation.
In the ProtecT trial, radical prostatectomy incurred the greatest degree of sexual dysfunction among all three treatment arms, with some recovery of function over time.3 The negative effect of radiotherapy on sexual function was greatest at 6 months, but sexual function then recovered somewhat and was stable thereafter. Sexual dysfunction also declined in the active monitoring group over time:

Bowel Dysfunction
Bowel dysfunction is typically low for patients undergoing radical prostatectomy or active surveillance, but may be a detrimental side effect among men undergoing radiation therapy. In the ProtecT trial, bowel function was worse in the radiotherapy group at 6 months than in the other groups but then recovered somewhat, except for the increasing frequency of bloody stools; bowel function was unchanged in the active monitoring and radical prostatectomy groups:3

Bowel dysfunction and rectal toxicity has improved with the utilization of hydrogel rectal spacers. Prior to radiation therapy, patients may have a hydrogel rectal spacer (SpaceOAR or the newly FDA approved Barrigel hyaluronic acid rectal spacer, which affords easier contouring; BioProtect is a biodegradable balloon commercially available in Europe and currently under review with the FDA) placed in a transperineal fashion in the fat between the rectum and Denonvilliers fascia. In the clinical trial assessing SpaceOAR hydrogel spacers, 114 patients were enrolled between 2010 and 2011, with 54 patients selected for a hydrogel injection before the beginning of radiation therapy.5 Patients were surveyed at various time-points with the EPIC prostate cancer questionnaire – among patients treated with a hydrogel spacer, mean bowel function and bother score changes of >5 points in comparison with baseline levels were found only at the end of radiation therapy (10-15 points; p < 0.01). Mean bowel bother score changes of 21 points at the end of radiation therapy, 8 points at 2 months, 7 points at 17 months, and 6 points at 63 months after radiation therapy were found for patients treated without SpaceOAR. In the clinical trial assessing Barrigel, there were 201 men randomized to either Barrigel (n = 136) or the control arm (n = 65). Overall, 98.5% of men treated with Barrigel met the primary endpoint of a 25% reduction in radiation to the rectum. Additionally, Barrigel reduced acute and long-term Grade 2+ bowel toxicity at 3 and 6 months compared to the control arm. These bowel quality of life results have given hydrogel spacers an option among patients considering radiation therapy.
Other health related effects
There is evidence that both radiotherapy and androgen deprivation therapy may contribute to the development of coronary heart disease, sudden cardiac death, myocardial infarction, and skeletal-related events such as fracture.6
Psychological Side Effects
Depression and Anxiety
Depression is the most common psychiatric comorbidity among cancer patients, including patients with prostate cancer. Ravi et al.7 previously utilized the SEER-Medicare database to assess the burden of mental health issues (anxiety, major depressive disorder, suicide) in patients with localized prostate cancer. Among 50,586 men >65 years of age without a diagnosis of mental illness, 20.4% men developed mental illness with a median 55-month follow-up. Interestingly, patients undergoing watchful waiting (29.7%) and radiation (29.0%) had a significantly increased incidence of mental illness compared to patients undergoing radical prostatectomy (22.6%; p<0.001). A systematic review of depression and anxiety in patients with prostate cancer identified 27 articles comprising 4,494 patients.8 The meta-analysis of prevalence rates identified pretreatment prevalence of depression of 17.27% (95%CI 15.06%-19.72%), on-treatment prevalence of 14.70% (95%CI 15.06%-19.72%) and post-treatment prevalence of 18.44% (95%CI 15.18%-22.22%). For anxiety, pretreatment prevalence was 27.04% (95%CI 24.26%-30.01%), on-treatment was 15.09% (95%CI 12.15%-18.60%) and post-treatment was 18.49% (95%CI 13.81%-24.31%). For patients undergoing active surveillance, nearly one third of patients (29%) report cancer specific anxiety in the year following diagnosis.9 Interestingly, over time, this anxiety decreased significantly over time.
There is also increasing evidence that androgen deprivation therapy for locally advanced and metastatic prostate cancer is associated with depression. A study from 2016 using SEER-Medicare data found that men that received ADT, compared with patients who did not receive ADT, had higher 3-year cumulative incidences of depression (7.1% v 5.2), inpatient psychiatric treatment (2.8% v 1.9%), and outpatient psychiatric treatment (3.4% v 2.5%).10 As follows is the cumulative incidence plot of time to diagnosis of depression by receipt of any androgen deprivation therapy:

As follows is the unadjusted cumulative incidence of time to inpatient psychiatric treatment by receipt of androgen deprivation therapy:
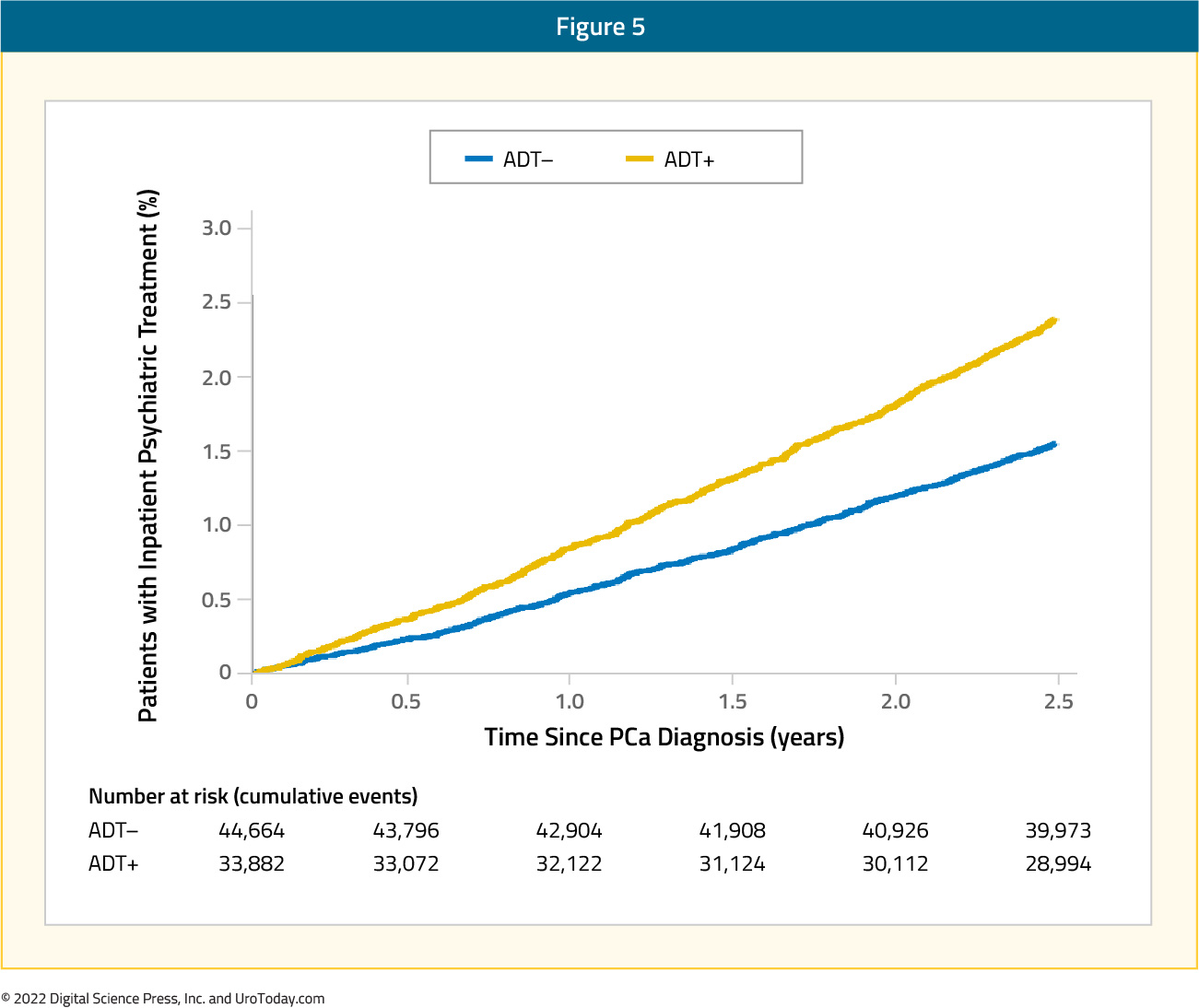
Furthermore, the risk of depression increased with duration of ADT, from 12% with ≤ 6 months of treatment, 26% with 7 to 11 months of treatment, to 37% with ≥ 12 months of treatment. A recent meta-analysis of 18 studies among 168,756 men found that ADT use conferred a 41% increased risk of depression (RR 1.41, 95%CI 1.18-1.70).11 These results were consistent when limiting the analysis to studies in localized disease (RR 1.85, 95%CI 1.20-2.85). Interestingly, this analysis did not find an association for continuous ADT with depression risk compared to intermittent ADT (RR 1.00, 95%CI 0.50-1.99).
Suicidal Risk
Patients with prostate cancer have been shown to be at increased risk of suicide across several population-level studies. In a SEER analysis assessing suicide risk among patients with genitourinary malignancies from 1988-2010, Klaassen et al.12 found an age-adjusted standardized mortality ratio (SMR) of 1.37 for patients with prostate cancer (95%CI, 0.99-1.86) Increasing age, metastatic disease and Caucasian race were risk factors for suicide among these patients. Interestingly, even patients >15 years after diagnosis were at increased risk of suicide compared to the general population (SMR 1.84, 95%CI 1.39-2.41). In an assessment of prostate cancer suicidal risk compared to individuals with other malignancies, Dalela et al.13 found that risk of suicidal death was no different in men with prostate cancer (1,165 [0.2%]) compared to men with other cancers (2,232 [0.2%]), However, within the first year of diagnosis, men with prostate cancer had an increased risk of suicide (ARR 3.98, 95%CI 3.02-5.23 0-3 months after diagnosis). Furthermore, men with non-metastatic prostate cancer who were Caucasian, uninsured, or recommended but did not receive treatment (HR vs treated 1.44, 95%CI 1.20-1.72) were at increased risk of suicidal death.
A meta-analysis of observational studies assessing incidence and risk factors of suicide after prostate cancer diagnosis was recently published.14 This study included 8 observational studies involving 1,281,393 men diagnosed with prostate cancer and 842,294 matched prostate cancer-free men. Guo et al. found an overall increased relative risk of suicide of 2.01 (95%CI 1.52-2.64) among men diagnosed with prostate cancer compared with those without prostate cancer during the first year after diagnosis, particularly during the first 6 months after diagnosis (RR 2.24, 95%CI 1.77-2.85). Additionally, prostate cancer patients were at an increased risk of suicide among men aged 75 years or older (RR 1.51, 95%CI 1.04-2.18) and for those treated with hormonal therapy (RR 1.80, 95%CI 1.54-2.12).
Until recently, all population-level studies assessing risk of suicide among prostate cancer patients have not accounted for psychiatric comorbidities at the time of diagnosis. This is important, considering that being unable to adjust for psychiatric comorbidities makes it impossible to assess the true risk associated with a prostate cancer diagnosis on suicidal risk. Klaassen et al.15 assessed all residents of Ontario, Canada diagnosed with either prostate, bladder or kidney cancer (1997-2014). Each patient was assigned a psychiatric utilization gradient (PUG) score in the five years prior to cancer diagnosis: 0 (none), 1 (outpatient), 2 (emergency department), 3 (hospital admission). Non-cancer controls were matched 4:1 to cancer patients based on sociodemographic variables and a marginal cause-specific hazard model was used to assess the effect of cancer on the risk of suicidal death. Among 191,068 patients included (137,699 prostate cancer, 29,884 bladder cancer, 23,485 kidney cancer), 109,154 (57.1%) were PUG score 0, 79,553 (41.6%) PUG score 1, 1,596 (0.84%) PUG score 2, and 765 (0.40%) PUG score 3. Patients with genitourinary cancer had a higher risk of dying of suicide compared to controls (HR 1.16, 95%CI 1.00-1.36). Specifically, among individuals with PUG score 0, those with cancer were significantly more likely to die of suicide compared to patients without cancer (HR 1.39, 95%CI 1.12-1.74).
Developing a Multidisciplinary Prostate Cancer Survivorship Program
The Commission on Cancer requires cancer centers to develop and implement processes to monitor formation and dissemination of a survivorship care plan for all cancer patients with stage I-III disease treated with curative intent, and to have this plan in place within 1-year of diagnosis of cancer and no later than 6 months after completing adjuvant therapy.16 Key components of a survivorship care plan are as follows:
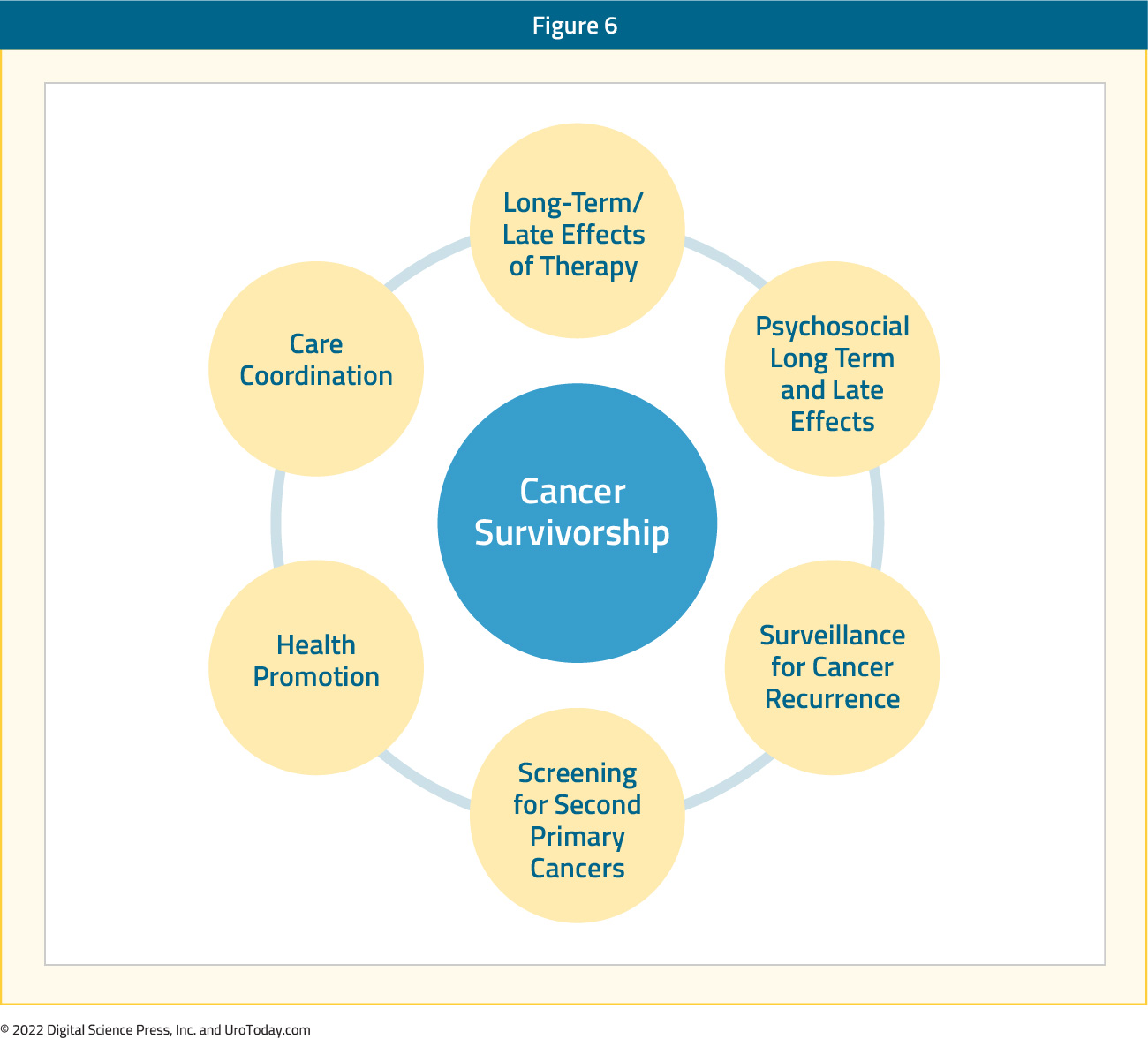
Guideline recommendations for prostate cancer survivorship have primarily been driven by the American Cancer Society and American Society of Clinical Oncology. The American Cancer Society noted in their guideline that survivorship should promote comprehensive follow-up care and optimal health and quality of life for the posttreatment prostate cancer survivor.17 The guidelines also address health promotion, surveillance for prostate cancer recurrence, screening for second primary cancers, long-term and late effects assessment and management, psychosocial issues, and care coordination among the oncology team, primary care clinicians, and non-oncology specialists. Subsequently, the American Society of Clinical Oncology Endorsement Panel reviewed the American Cancer Society guidelines, endorsing these guidelines with the following recommendations:18
- Measure PSA level every 6 to 12 months for the first 5 years and then annually, considering more frequent evaluation in men at high risk for recurrence and in candidates for salvage therapy
- Refer survivors with elevated or increasing PSA levels back to their primary treating physician for evaluation and management
- Adhere to American Cancer Society guidelines for the early detection of cancer
- Assess and manage physical and psychosocial effects of prostate cancer and its treatment
- Annually assess for the presence of long-term or late effects of prostate cancer and its treatment
There are several screening tools to assess for quality of life, depression, and suicidal risk. A study from 2017 assessed differences in the scores, relative severity and major depressive disorder from three standardized self-report scales for depression in prostate cancer patients [The Hospital Anxiety and Depression Scale Depression subscale (HADS-D), the Self-rating Depression Scale (SDS) and the Patient Health Questionnaire (PHQ-9) for depression].19 Among 138 prostate cancer patients, despite significant correlations between the total scores from the three scales, severity classification differed across the three scales. Furthermore, there was considerable underestimation of depression by the HADS-D compared to the PHQ-9, and a similar tendency for the SDS. This study highlights that scale construction and depression items included can produce different results across scales, making inter-study comparisons difficult. Despite these findings, we recommend that at minimum oncologists should be using at least one depression index to assess patient well-being at each clinic visit.
In addition to the aforementioned HADS-D, SDS, and PHQ-9 metrics, the National Comprehensive Cancer Network (NCCN) provides a guideline for identifying and explaining risk factors in patients with cancer, in addition to providing a “distress thermometer”

The NCCN defines distress, in the setting of cancer, as a multifactorial emotional experience of a psychological, social, and/or spiritual nature that may interfere with the ability to cope effectively with the diagnosis.20 Distress can range from sadness and fear to more disabling symptoms such as anxiety and depression. Furthermore, the time periods at which patients are at increased vulnerability begin with the realization of a suspicious symptom, all the way through to failure/disease recurrence and near the end of life. The NCCN recommends screening all patients for distress to recognize, monitor, and treat patients effectively.20
Previous work has also suggested that screening for depression and erectile dysfunction may be a way to decrease suicidal risk among prostate cancer patients.21 A proposed algorithm allows for an initial evaluation with the EPIC-CP and PHQ-9 tools to assess for health-related quality of life and depression, respectively. If the EPIC-CP or PHQ-9 are negative for depression or erectile dysfunction, these tools should still be used at each visit to regularly evaluate patients. If EPIC-CP or PHQ-9 suggest problems with depression or erectile dysfunction, then an 8-question suicidal ideation questionnaire (adapted from Recklitis et al.22 ) should be completed. If the suicidal ideation questionnaire demonstrates any level of suicidal ideation, clinicians should make an urgent referral for psychiatric evaluation. This is particularly true when the patient has the concomitant high-risk suicidal risk profile of being elderly, white, single, or with high-risk or disease progression. Given that, at maximum, the patient must answer a 27-point composite questionnaire, this should be feasible in the busy clinical setting and can be provided to the patient at appointment check-in and completed in the waiting room before the physician-patient encounter:
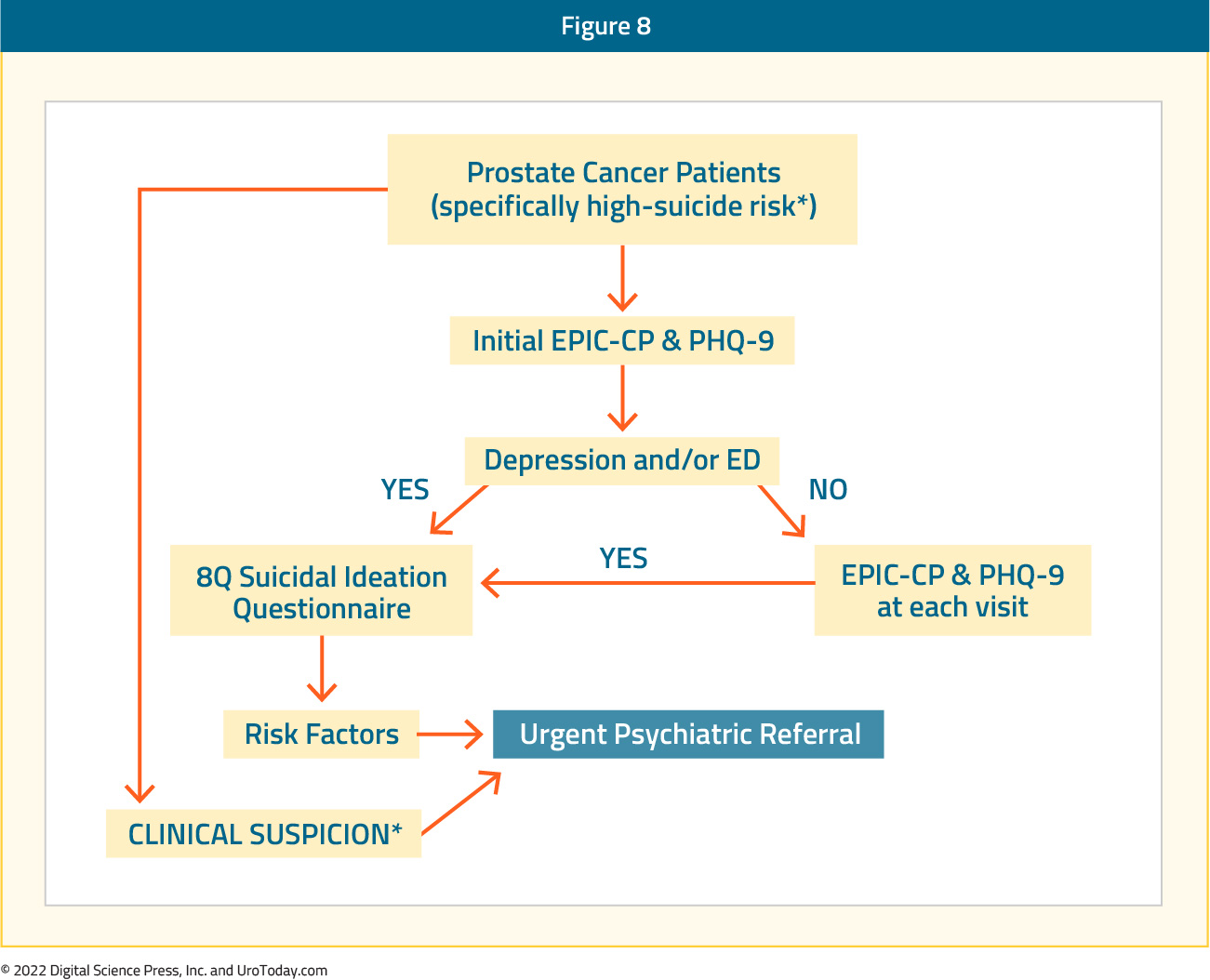
Regardless of the results from these screening tools, if any member of the healthcare team has an index of suspicion for suicidal ideation, the physician should immediately make a referral for psychiatric evaluation.
Key Prostate Cancer Survivorship Team Members
In order to provide holistic, comprehensive prostate cancer survivorship care, there are a number of important members that need to be part of a survivorship team. Indeed, the “quarterback” is likely to be the patient’s oncology provider (whether a urologist, radiation oncologist, and/or medical oncologist). Primarily secondary to the sexual side effects of localized treatment for prostate cancer, many cancer centers now have fellowship trained urology experts that see these patients concomitantly with the oncologist. There are a variety of treatment options offered, including oral PDE-5 inhibitors (sildenafil, tadalafil, etc), intracavernosal injection therapy, and penile prosthetics. Additionally, these urologists are typically well-versed in addressing post-treatment urinary incontinence and are able to offer the patients urethral slings and artificial urinary sphincters. In our opinion, this duo (oncology provider and urologist specialized in post-prostatectomy incontinence and erectile dysfunction) is crucial to providing adequate prostate cancer survivorship care. A psycho-oncology team is also important to the prostate cancer survivorship team, being readily available to intervene when patients are screened for distress, depression, suicidal ideation, etc. Finally, there are many other key members of the prostate cancer survivorship team including, but not limited to, nurse practitioners, physician assistants, social workers, case managers, nurse navigators, etc. Recent studies have suggested that the level of patient/partner satisfaction in patient-centered survivorship programs is high.23 The typical prostate cancer survivorship journey at our institution is detailed as follow:

Conclusions
With 3 million men in the United States living with prostate cancer, survivorship programs are now mandated by the Commission on Cancer and play an integral role in health and well-being of men with prostate cancer. In addition to physical side effects of treatment that should be addressed at each clinic visit, there are crucial psychiatric side effects, including depression, anxiety, and suicidal ideation, that should be screened for and recognized by all members of the health care team. In order to provide comprehensive, holistic prostate cancer survivorship care, it is important for key team members to be specialized in their specific role in order to meet the needs of prostate cancer survivors.Written by:
- Zachary Klaassen, MD, MSc, Assistant Professor of Surgery/Urology Medical College of Georgia – Augusta University Augusta, GA
- Sherita A. King, MD, Assistant Professor of Surgery/Urology Medical College of Georgia – Augusta University Augusta, GA


