Background
Kidney cancer represents 5% of all new cancer diagnoses in the United States, with approximately 64,000 new cases and 14,970 deaths in 2018.1,2 The most common type of kidney cancer is renal cell carcinoma (RCC) and the most common histologic subtype of RCC is clear cell RCC, accounting for over 80% of cases.3 RCC is more common in men than women and typically occurs in the sixth to eighth decade of life.1 Localized kidney cancer can often be cured with definitive surgery, with 5-year survival reaching over 90%.4 However, for patients with advanced disease, 5-year survival remains poor at 11.7% and much progress is needed to develop novel therapies for advanced RCC.4Risk Stratification
The current treatment paradigm for metastatic clear cell RCC requires stratification of patients into favorable, intermediate, or poor risk disease. Several validated models for risk stratification exist, including the International Metastatic Renal Cell Carcinoma Database Consortium model (IMDC)5 and Memorial Sloan Kettering Cancer Center model (MSKCC).6 Both criteria include time from diagnosis to systemic treatment of less than one year, performance status, as well as hemoglobin and calcium (Table 1). The main differences between these models are that the MSKCC model includes LDH and the IMDC includes neutrophil and platelet count as unique risk factors. Patients with no risk factors fall into the favorable risk group, patients with one to two risk factors are in the intermediate risk group, and those with three or more risk factors are in the high-risk group for both prognostic models. Contemporary clinical trials have found that drug effectiveness may vary depending on risk stratification which has led the FDA to approve some therapies for only certain risk groups.7 Thus, risk stratification is important for the clinician, not only for discussing prognosis with patients, but also for treatment selection.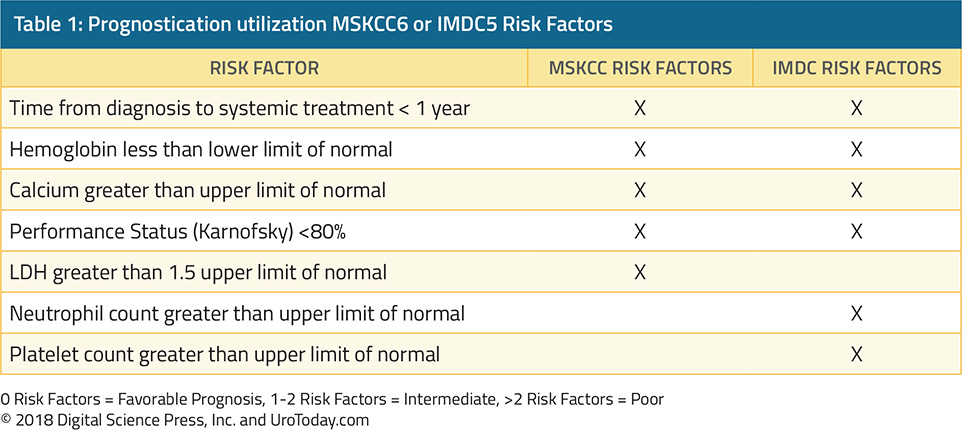
Favorable Risk Patients
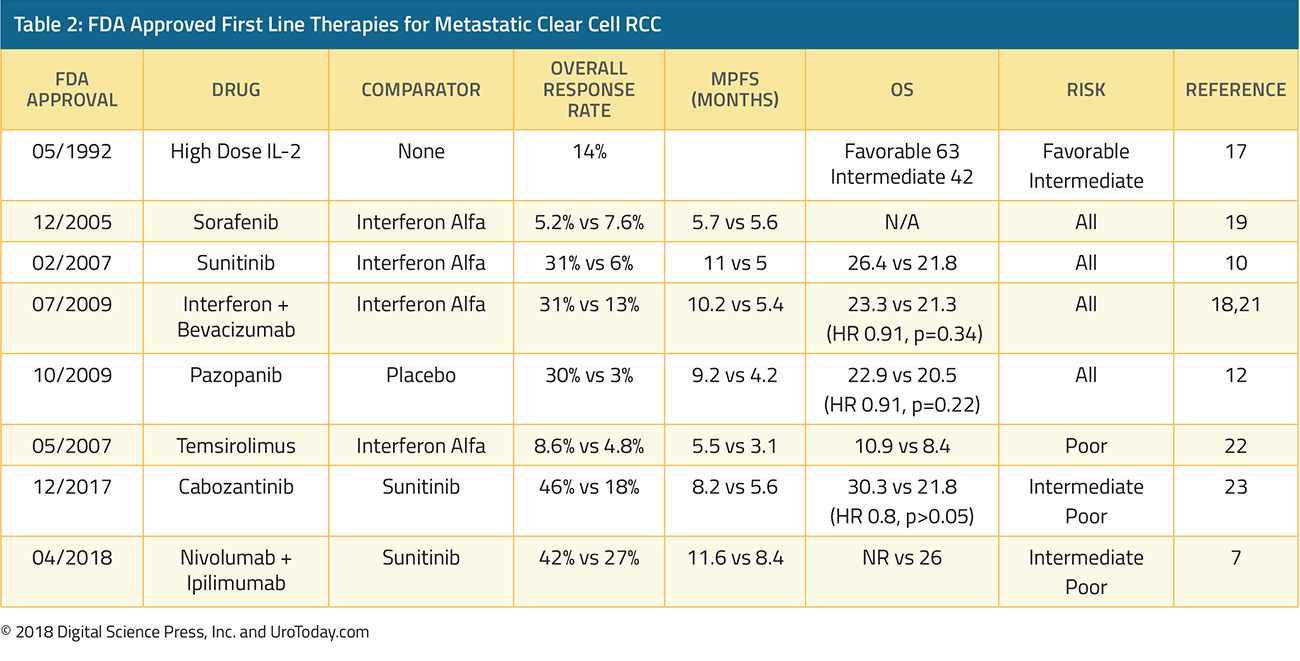
For patients with no adverse risk factors, sunitinib and pazopanib are the preferred first line treatment options, recommended by both the National Comprehensive Cancer Network (NCCN) as well as the European Association of Urology (EAU).8,9 Sunitinib is an oral multikinase inhibitor which targets vascular endothelial growth factor receptors (VEGFR) and platelet-derived growth factor receptors (PDGFR). A phase III trial comparing sunitinib to interferon-α showed that sunitinib significantly improved median progression free survival (11 months vs 5 months) and had a higher overall response rate as well when compared with interferon alfa (31% vs 6%).10 Sunitinib also demonstrated longer overall survival in a follow up study, 26.4 months vs 21.8 months.11 Severe adverse events (grade 3-4 toxicities) were minimal, and the most common adverse events were diarrhea, fatigue, and nausea. Hypertension was one notable side effect of sunitinib, which was not seen with interferon alfa. Based on the data above, sunitinib was granted FDA approval in 2007 and has been the benchmark for many future clinical trials in the mRCC space.Pazopanib is another oral multikinase inhibitor, which targets VEGFR-1,2,3, PDGFR- and , and c-KIT.12 Pazopanib was established as a safe and efficacious therapy for mRCC based on a large randomized, placebo controlled trial in patients who were treatment naive or cytokine pretreated.12 In this trial of 435 patients, pazopanib increased progression-free survival (9.2 months vs 4.2 months) compared with placebo, both in the treatment naïve cohort as well as the cytokine pretreated cohort. The objective response rate to pazopanib was 30% and pazopanib was well tolerated. Unique toxicities of pazopanib included notable grade 3 hepatotoxicity 30% of patients had elevated ALT and 21% had elevated AST. These results granted pazopanib FDA approval in October 2009. A subsequent phase III study (COMPARZ) with 1,110 patients compared sunitinib to pazopanib, and pazopanib was found to be noninferior to sunitinib with respect to progression-free survival and overall survival.13 Median overall survival was 28.4 months in the pazopanib arm compared with 29.3 months in the sunitinib arm. In terms of safety, similar percentages of patients in both sunitinib and pazopanib experienced dose interruptions of one week or greater, 44% and 49% respectively. Patients in the pazopanib arm more frequently discontinued therapy based on abnormal liver function tests. Patients taking sunitinib had a higher risk of abnormal hematologic labs including leukopenia, thrombocytopenia, neutropenia, and anemia.
Additional FDA approved therapies for first line management of favorable risk patients include high dose interleukin 2 (HD IL-2)14-17, interferon plus bevacizumab18, and sorafenib.19 These therapies are less commonly used given their tolerability and toxicity profiles. HD IL-2 deserves special mention here given its ability to induce complete responses in a small subset of patients. A recent abstract describing overall survival from the PROCLAIM database show that of favorable risk patients, median overall survival is 63.3 months and 2 year overall survival is 77.6%.14 Of course, patients who are given HD IL-2 are a very carefully selected robust cohort, and cross-trial comparisons are challenging to make.
Intermediate and Poor Risk
Per the IMDC and MSKCC prognostic models, patients with one or two risk factors are classified as intermediate risk, and poor risk if they have three or more risk factors. Currently, the two major newcomers in this space are cabozantinib and the combination of ipilimumab and nivolumab. Cabozantinib is a multikinase inhibitor of VEGFR, MET, and AXL.20 In the phase II CABOSUN study, 157 intermediate or poor risk patients were randomized to cabozantinib or sunitinib. In this population, cabozantinib increased median progression free survival (8.2 months vs 5.6 months) and improved overall response rate (33% vs to 12%) compared with sunitinib. Both sunitinib and cabozantinib had about a 67% grade 3/4 adverse event rate and had a similar toxicity profile, including fatigue, hypertension, and diarrhea. Sunitinib had a lower incidence of hand foot syndrome and weight loss compared with cabozantinib, but higher rates of neutropenia and thrombocytopenia. Given this data, Cabozantinib obtained FDA approval for the front-line treatment of mRCC in December 2017.The newest therapy to obtain FDA approval is the combination checkpoint inhibitor duo Ipilimumab and Nivolumab (Ipi/Nivo); it was approved in April of 2018, based on the results of CheckMate214.7 CheckMate 214 was a randomized, open-label trial comparing sunitinib with Ipi/Nivo. 1096 patients were enrolled, of which 847 were intermediate or poor risk. This study had a coprimary endpoint of overall survival, objective response rate, and progression free survival among patients who were intermediate or poor risk. The overall response rate was 42% with ipi/nivo vs 27% with sunitinib, with a complete response rate of 9% vs 1%. Median overall survival has not been reached with ipi/nivo vs 26 months for sunitinib. Progression free survival was 11.6 months for ipi/nivo compared with 8.4 months for sunitinib. 46% of patients receiving ipi/nivo experienced grade 3/4 toxicities compared with 63% of patients receiving sunitinib. The most common grade 3/4 adverse events with ipi/nivo was fatigue, diarrhea, and elevated lipase compared with hypertension, hand-foot syndrome, and increased lipase with sunitinib. 35% of patients required high dose corticosteroids for immune related toxicities with ipi/nivo. Â With this data, the European Association of Urology has recommended that ipi/nivo be the new standard of care for patients with intermediate and poor risk disease, and the NCCN has also listed ipi/nivo as a category 1, preferred treatment option for patients with intermediate and poor risk disease.8,9
Future Therapies
Front-line treatment options for mRCC are rapidly evolving.24 Data shown at ASCO and GU ASCO has demonstrated that antiangiogenic agents in combination with checkpoint inhibitors may prolong progression-free survival when compared with kinase inhibitors.25 Several phase III trials exploring this hypothesis are now underway (Table 3). IMmotion 151 is a randomized phase III trial comparing the combination of atezolizumab + bevacizumab vs sunitinib. Progression-free survival was 11.2 months in the intention to treat analysis for patients atezolizumab and bevacizumab, compared with 8.4 months in the sunitinib arm. JAVELIN Renal 100 is investigating avelumab in combination with axitinib and phase I results show a promising overall response rate of 54.5% out of 55 patients.26 KEYNOTE-426 is investigating pembrolizumab in combination with axitinib, compared with sunitinib alone.27 These trials are important and exciting for our patients with mRCC and their future results may alter the standard of care for frontline mRCC.
Published Date: January 29th, 2019


