Advances in the radiologic investigation of prostate cancer have occurred in two domains: local imaging of the prostate to guide diagnosis and local therapy and whole-body imaging to detect and guide treatment of metastatic disease.
Diagnosis and assessment of the prostate
Following initial approaches guided by digital palpation per rectum (so-called, finger-guided biopsies),1 prostate cancer diagnosis has been guided by transrectal ultrasonography (TRUS) since the early 1990s. While this approach rapidly became the gold standard approach to prostate cancer diagnosis,2 there are numerous limitations to the use of TRUS. Most notably, standard ultrasonography is unable to reliably distinguish between benign and malignant prostate tissue. As a result, rather than being able to target suspicious regions, TRUS-guided biopsies are systematic with sampling occurring by chance in the absence of clear visible hypoechoic suspicious areas. Additionally, the transrectal approach, particularly without further radiographic image to guide, consistently under-samples regions of the prostate, including the anterior and apex.3 Due to these limitations, TRUS-guided systematic prostate biopsy can miss up to 20% of clinically significant prostate cancer, resulting in underdiagnosis and undertreatment4 while at the same time detecting a relatively high percentage of clinically insignificant prostate cancer (Gleason grade group [GGG] 1), which may result in overtreatment.5
To address the limitations of this approach, novel imaging approaches have been sought. The first among these, and most widely adopted, is multiparametric magnetic resonance imaging (mpMRI). Using the Prostate Imaging – Reporting and Data System (PI-RADS v2) approach, mpMRI allows for better characterization of the prostate and any lesions within. While initial MRI-based morphological assessment using T1-weight and T2-weighted pulse sequences provided the ability to perform locoregional staging for previously diagnosed prostate cancer,6 the use of more advanced techniques including functional and physiologic assessment with diffusion-weighted imaging (DWI), apparent-diffusion coefficient (ADC) mapping, dynamic contrast enhancement (DCE), and proton spectroscopy have allowed for a non-invasive risk stratification of the likelihood of prostate cancer. This has allowed for the use of mpMRI to guide prostate biopsy, either directly with in-bore biopsy or more commonly using a fusion device platform.7
While initially used in the evaluation of patients with elevated PSA levels with previous negative prostate biopsy,8 mpMRI is now routinely used in the evaluation of patients with elevated PSA prior to initial biopsy both for risk stratification and to guide biopsy. A recent systematic review and meta-analysis from Kasivisvanathan and colleagues suggested that mpMRI targeted biopsy detects more clinically significant prostate cancer than standard TRUS-guided systematic biopsy alone and requires fewer prostate cores to do so; that the question of whether to include systematic biopsy along with mpMRI targeted biopsy remains controversial; and that the omission of the systematic biopsy risks missing the diagnosis of clinically significant disease in approximately 13% of men while inclusion of systematic biopsy increases the likelihood of diagnosing clinically insignificant prostate cancer.9
Moving even further forward in the diagnostic algorithm, MRI has recently been examined as an initial screening test, rather than PSA, for prostate cancer. While there are ongoing trials assessing these, the prospective population-based IP-1 PROSTAGRAM study was published in JAMA Oncology this year.10 This study enrolled 408 men aged 50 to 69 years who underwent prostate cancer screening using a PSA test, MRI, and ultrasonography. The authors compared the proportion of men with “abnormal” screening results, as well as the number with clinically significant and clinically insignificant prostate cancer. The authors found that positive MRIs (defined as 3-5 on a 5-point scale) were more common (17.7%) than elevated PSA tests (defined as 3 ng/mL or greater; 9.9%), though the proportion of abnormal results was even higher for ultrasonography (23.7%), with each comparison being statistically significant. However, when a threshold of 4-5 on a 5-point scale was used to define abnormal imaging results, the proportion with abnormal screening tests was similar (10.6% for MRI and 12.8% for ultrasound). Elevated PSA tests detected 7 clinically significant and 6 clinically insignificant cancers. In contrast, MRI detected 14 and 11 clinically significant cancers using abnormal definitions of 3-5 and 4-5, respectively, while detecting 7 and 5 clinically insignificant cancers, respectively. Similarly, ultrasonography detected 9 and 4 clinically significant cancers using abnormal definitions of 3-5 and 4-5, respectively, while detecting 13 and 7 clinically insignificant cancers, respectively. These data, while requiring replication, suggest that MRI may be a preferable prostate cancer screening test compared to PSA.
Despite this promise, there remain limitations to the widespread use of MRI. These include only a moderate inter-reader reproducibility of mpMRI, the lack of standardization of targeted biopsy, and cost-effectiveness concerns in certain jurisdictions. In a risk-adapted approach informed by PSA results and polygenic risk profiles, data from the UK has suggested that MRI prior to initial biopsy is likely to be cost-effective.11
Even more recently, high-resolution micro-ultrasound has emerged as a novel imaging modality for prostate cancer. This approach leverages many of the benefits of MRI along with those of ultrasonography. The very fine resolution (approximately 70 µm) of high-resolution micro-ultrasound allows for visualization of alterations in ductal anatomy and cellular density consistent with prostate tumors.12 In early experiences, high-resolution micro-ultrasound has demonstrated an ability to detect clinically significant cancers that were not apparent on either traditional TRUS or mpMRI.13 Thus, from a diagnostic and localization perspective, high-resolution micro-ultrasound offers much of the benefit of mpMRI. However, in addition, in contrast to mpMRI, high-resolution micro-ultrasound has the advantage of providing real-time imaging results, a finding that authors from the Cleveland Clinic demonstrated was associated with a relative increase in prostate cancer detection of 26.7%.13 While mpMRI is helpful for localizing intra-prostatic lesions, biopsy requires fusion to a standard ultrasound image either cognitively or through registration, or in-bore biopsy which is rarely performed due to cost, time, and inconvenience. In contrast, high-resolution micro-ultrasound offers similar discriminative ability while facilitating real-time prostate imaging and guiding biopsy.
Aggregate data from early clinical experience at multiple centers suggests that high-resolution micro-ultrasound has comparable or increased sensitivity for clinically significant prostate cancer compared with mpMRI and comparable or slightly reduced specificity.12
Distant staging
While mpMRI has revolutionized imaging of the prostate and substantially changed the diagnostic algorithm for prostate cancer, perhaps even greater changes have occurred in the imaging for distant disease.
Initially, radiographic diagnosis of bony prostate cancer metastasis was made on the basis of plain radiographs. However, this approach is limited as extensive bone mineral loss (exceeding 30-50%) may be required before such changes are radiographically apparent.14 Following plain projectional radiography, skeletal scintigraphy was the next imaging modality widely adopted for the assessment of bony metastases in patients with prostate cancer. To date, it remains widely utilized and is currently recommended, along with abdominal and pelvic computed tomography (CT), for the staging of patients according to many guideline bodies. Skeletal scintigraphy, when performed in patients with known cancer in the absence of bony pain, has a sensitivity of 86% and specificity of 81% for the detection of metastatic lesions.14 As with any imaging modality, these characteristics differ somewhat on the basis of the patient population being tested (i.e. the pre-test probability or population-based disease prevalence). Among patients with prostate cancer, PSA levels are predictive of the likelihood of a positive bone scan. Across a number of different cancers, Yang et al. found that bone scintigraphy had a specificity of 81.4% and sensitivity of 86.0%, on a per-patient basis, for the detection of bony metastases.15 In addition to a bone scan, computed tomography has been utilized for the assessment of nodal metastatic disease, visceral disease, and bony metastasis. CT is highly sensitive for both osteoblastic tumors (such as prostate cancer) and osteolytic lesions in the cortical bone but is less sensitive in tumors that are restricted to the marrow space.14 As a result, CT is of relatively limited utility as a screening test for bony metastasis due to relatively low sensitivity (73%) despite excellent specificity (95%) – numbers based on a large-scale meta-analysis from Yang and colleagues.15 For this reason, conventional staging recommendations for patients with prostate cancer include bony scintigraphy for the detection of bony lesions along with CT for identification of nodal/visceral lesions and correlation of any bony lesions.16
In addition to its role in the local staging of the prostate and guidance of prostate biopsy, mpMRI may also assist with evaluation for distant metastatic disease. Routine pelvic/prostate MRI typically allows for assessment of local/regional nodal involvement including obturator and external iliac nodal chains. As a result, pelvic/prostate mpMRI may substitute for abdominal pelvic CT for nodal staging. However, the high soft-tissue contrast and high spatial resolution afforded by MRI call also allow for the identification of bony metastasis in marrow spaces much early than would be apparent based on CT scan.15 Further, the use of T1-weighted sequences and STIR sequences can allow for adequate assessment for bony metastasis without the need for intravenous contrast agents; the use of MRI for staging also does not require the use of ionizing radiation. Thus, whole-body MRI may be considered for the identification of distant metastatic disease. Additionally, MRI with contrast has become the imaging modality of choice for the evaluation of liver metastases.17 Thus, this approach may be particularly valuable in patients at high risk of visceral metastatic disease.
However, the large most recent, and most meaningful recent change in prostate cancer imaging has come with the increased utilization of molecularly-targeted functional imaging. Traditional positron emission tomography (PET) imaging utilizing fluorodeoxyglucose (FDG) is not typically effective in the initial diagnosis of prostate cancer metastasis owing to the relatively low metabolic activity associated with the disease. However, at least four other PET imaging approaches have been assessed and employed in patients with prostate cancer including 18F-NaF PET/CT, choline-based PET/CT, fluciclovine (Axumin) PET/CT, and PSMA-targeted PET/CT.18
For the most part, these were initially evaluated in the setting of recurrent disease but have also been evaluated in primary staging. Choline-based PET/CT has been available for quite some time but is geographically limited and therefore not widely used. However, compared with conventional CT and bone scan, choline-based PET/CT has significantly higher sensitivity for the diagnosis of metastatic lesions at the time of biochemical recurrence.18
Most widely used in the United States is fluciclovine PET/CT (also known as Axumin PET/CT) which utilizes the proliferation of tumor cells for localization. Fluciclovine (18F-FACBC; 1-amino-3-fluorine 18F-flurocyclobutane-1-carboxylic acid) is a synthetic amino acid analogue with the advantage of negligible renal uptake and no activity in the urinary tract.19 However, non-specific prostate uptake limits its utility in identification of primary prostate tumors due to an inability to distinguish from benign prostatic inflammation. Instead, fluciclovine-PET/CT has proven efficacy in the detection of recurrent prostate cancer with biochemical recurrence following local therapy, with a sensitivity of 90% and specificity of 40% (higher in distant, 97%, and nodal disease, 55%, than locally).20 Compared to choline-PET/CT, fluciclovine-PET/CT demonstrated lower false negatives and false-positive rates in patients with biochemical recurrence.21,22 The recently published EMPIRE-1 study is among the first studies to show an improvement in clinical outcomes with the use of molecularly targeted imaging.23 This single-center, open-label phase II/III randomized controlled trial enrolled men with biochemical recurrence and negative conventional imaging following radical prostatectomy for prostate cancer. Patients were randomized to receive radiotherapy on the basis of conventional imaging and pathologic characteristics alone or in combination with 18F-fluciclovine-PET/CT. Unlike many other studies that have utilized management changes as a proxy for clinical utility, this study examined three-year event-free survival. Among 165 randomized patients over a median follow-up of 3.5 years, the three-year event-free survival was significantly higher among patients who received radiotherapy informed by 18F-fluciclovine-PET/CT (75.5%, 95% confidence interval 62.5-84.6%) as compared to conventional imaging (63.0%, 95% confidence interval 49.2-74.0%) with a statistically significant difference (absolute difference 12.5%, 95% confidence interval 4.3-20.8%, p=0.003). After multivariable adjustment, the use of 18F-fluciclovine-PET/CT to guide radiotherapy decisions was significantly associated with event-free survival (hazard ratio 2.04, 95% confidence interval 1.06-3.93). Toxicity was similar been these approaches.
Finally, receptor-targeted PET imaging has recently been examined, most notably, prostate-specific membrane antigen (PSMA) -based PET/CT. PSMA is a type II transmembrane glycoprotein that functions as a zinc metalloenzyme and is found on prostatic epithelium. In normal prostate tissue, PSMA expression and localization focus on the cytoplasm and apical side of the epithelium surrounding prostatic ducts. However, during prostate carcinogenesis, PSMA is transferred to the luminal surface of the ducts. PSMA is highly expressed (100-1000 fold normal) in the vast majority of prostate cancer cells, including in patients with advanced disease, in those with castration-resistant disease, and in those with poorly differentiated disease. Further, PSMA has a high internalization rate into prostate cancer cells. The ratio of PSMA to its truncated isoform (PSM’) is proportion to tumor aggressivity. The most well-examined PSMA based approach is 68Ga-PSMA-PET/CT though others including 18F-DCFPyL have been recently examined and are gaining clinical relevance. While there are many studies examining these approaches, we will seek to highlight particularly practice changing or informing studies.
As alluded to above, PSMA-PET/CT was initially assessed in patients with biochemical recurrence based on the premise that more sensitive imaging may allow for earlier detection of disease, thus focusing on men with PSA less than 2 ng/mL. In this context, 68Ga-PSMA-PET/CT demonstrated superior detection rates of metastatic disease (56%) compared with fluciclovine-PET/CT (13%).24 This benefit was consistent in detecting pelvic nodal disease and extrapelvic disease. PSMA-based PET/CT demonstrated particular benefit in the evaluation of patients with low absolute PSA levels.
68Ga-PSMA-PET/CT was then assessed for initial tumor staging in the proPSMA study published in the Lancet25 and presented at the EAU 2020 Annual Meeting. This was a multi-center, two-arm randomized controlled trial among men with histologically confirmed prostate cancer who were being considered for curative intent radical prostatectomy or radiotherapy. To be eligible for inclusion, men must have had at least one high-risk factor including PSA greater than or equal to 20 ng/mL, ISUP grade group 3-5, or clinical stage T3 or greater. Patients who had undergoing staging investigations (apart from prostate MRI) within 8 weeks prior to randomization were excluded.
Following enrollment, patients were randomly assigned in a 1:1 ratio to either conventional imaging performed using bone scan and CT or PSMA PET/CT. Randomization was stratified according to center. Patients who were randomized to conventional imaging underwent abdominopelvic CT scan with contrast as well as technetium-99m bone scan with SPECT CT of chest, abdomen, and pelvic in keeping with the standard of care. These investigations were assessed in aggregate to determine the presence of findings of interest. For patients randomized to PET/CT, gallium-68 PSMA-11 PET/CT was performed. In patients who had fewer than 3 unequivocal sites of metastasis, cross-over imaging for confirmation was performed within 14 days. Confirmatory testing following imaging was performed at the discretion of the treating physician and included biopsy confirmation.

The primary study outcome was the accuracy of first-line diagnostic imaging for the identification of either pelvic nodal or distant metastatic disease. Accuracy was assessed using the area under the curve (AUC) of the receiver operating characteristic (ROC) curve. The reference standard was a composite panel of histopathology, imaging, clinical, and biochemical characteristics.
Between 2017 and 2018, the authors randomly assigned 302 patients of whom 300 received assigned first-line imaging. In keeping with the prostate cancer population, median age was 68 years, 293 men had ISUP grade 3 or higher, 65 had PSA 20 ng/mL or higher, and 82 had clinical stage T3 or T4. 96% (146) of men assigned to conventional imaging underwent subsequent second-line PSMA PET-CT. Assessment of the reference standard was possible in 295 (98%) of men, including 87 of whom had evidence of nodal or distant metastasis. Of these, hard criteria were used to define disease in 20 men.
In the primary outcome assessment, PSMA PET-CT had a 27% absolute greater AUC for accuracy compared to conventional imaging (95% confidence interval 23-31): 92% (95% CI 88-95%) vs 65% (60-69%). Conventional imaging had both a lower sensitivity (38% vs 85%) and also a lower specificity (91% vs 98%).
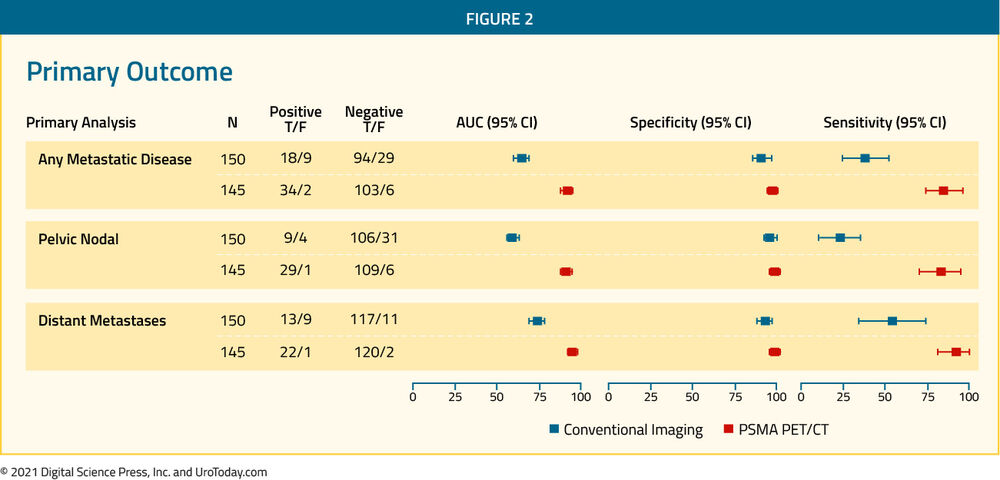
The authors performed a sensitivity analysis in which all lesions rated as equivocal were considered positive. This changed the results only marginally with an absolute difference of 28% (95% confidence interval 23-33%). These results were also consistent in subgroups of patients with pelvic nodal disease and those with distant metastasis. Further posthoc subgroup analysis showed incremental benefit for PSMA PET-CT in men with GGG4-5 disease, those with GGG less than or equal to 3, and those with a PSA of 20 ng/mL or greater. Further, equivocal findings were more common in men undergoing conventional imaging (23%) compared to those undergoing PSMA PET-CT (7%).
Prior to treatment, the results of conventional imaging studies resulted in treatment change for 23 men (15%, 95% confidence interval 10-22) while the results of PSMA PET-CT resulted in treatment change for 41 (28%, 95% confidence interval 21-36). These changes included both a transition from curative intent to palliative intent treatment in 20 patients (14%) and also a change in treatment approach in 22 (14%). These data demonstrate the clinical utility of utilizing PSMA PET-CT in this clinical space.
Further, conventional imaging was associated with a higher radiation dose (19.2 mSv compared to 8.4 mSv; absolute difference 10.9 mSv, 95% confidence interval 9.8-12.0 mSv0. PSMA PET-CT was not associated with any adverse events and reporter agreement was high for both nodal (kappa 0.87, 95% confidence interval 0.81-0.94) and distant metastatic disease (kappa 0.88, 95% confidence interval 0.94-0.92).
On December 1, 2020, the FDA approved 68Ga PSMA PET-CT for staging in men with prostate cancer with suspected metastasis before initial definitive therapy and in men with biochemical recurrence, although the approval is, at this time, limited to UCLA and UCSF.
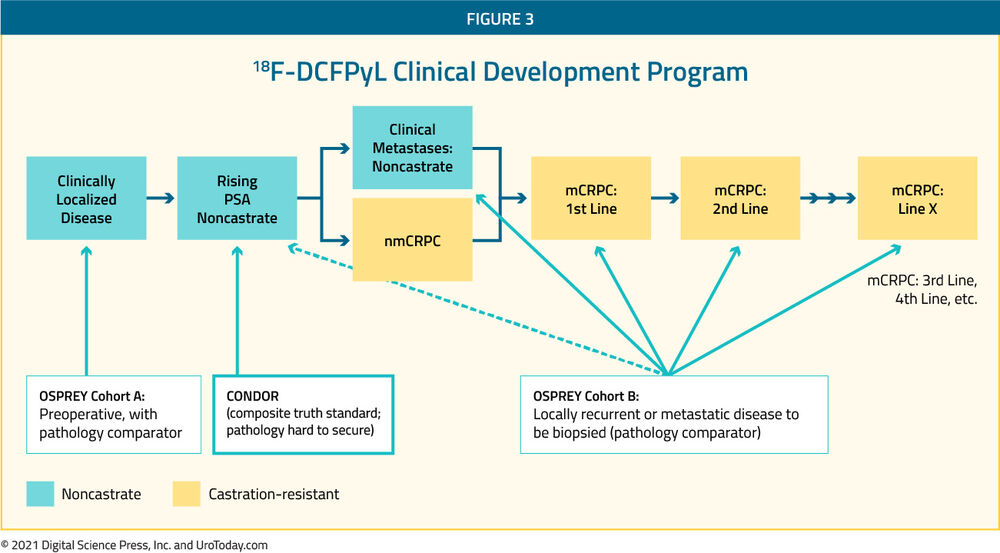
The second PSMA-based radiotracer which has recently seen considerable interest is 18F-DCFPyL. The developmental program for this tracer has spanned the spectrum of prostate cancer from initial staging of patients with high-risk disease who are planned for radical prostatectomy and pelvic lymphadenectomy through to mCRPC in the CONDOR26 and OSPREY27 studies.
The CONDOR study focused on patients with biochemically recurrent disease in which the authors recruited men aged 18 years and older with rising PSA after definitive therapy and negative or equivocal standard of care imaging (e.g., CT/MRI, bone scintigraphy, or F-18 fluciclovine).26 The authors undertook by PyL-PET/CT using s single 9 mCi (333 MBq) ± 20% dose of PyL, followed by PET/CT 1-2 hours later. Patients with positive 18F-DCFPyL-PET/CT scans based on local interpretation were scheduled for follow-up within 60 days to verify suspected lesion(s) using a composite SOT. As their primary outcome of interest, the authors assessed the correct localization rate (CLR), defined as the percentage of pts with a 1:1 correspondence between at least one lesion identified by PyL-PET/CT and the composite standard of truth: pathology, correlative imaging, or PSA response, in descending order of priority. The trial was successful if the lower bound of the 95% confidence interval for CLR exceeded 20% for at least two of three independent, blinded central 18F-DCFPyL-PET/CT reviewers.
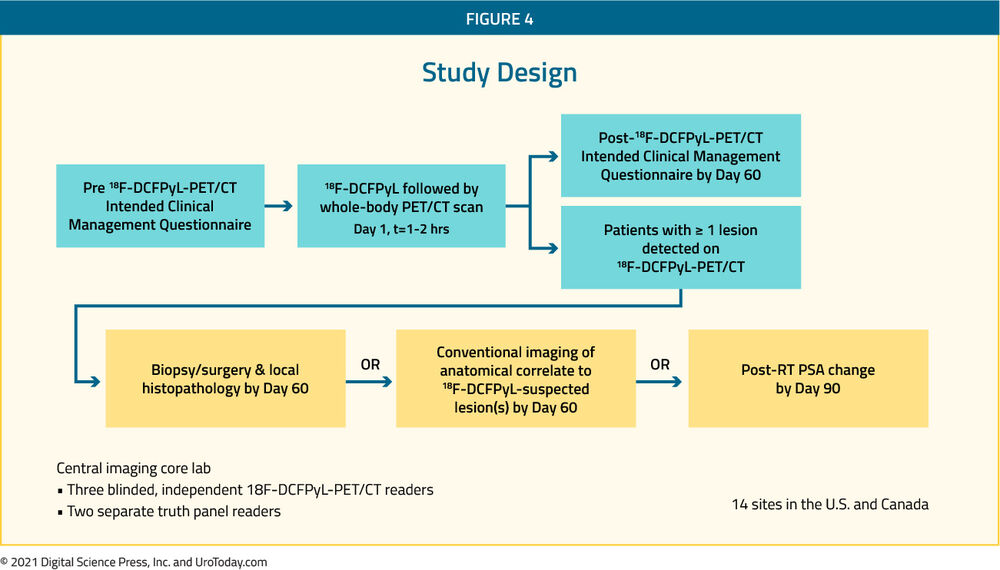
The authors accrued 208 men who met inclusion criteria. Median PSA in this cohort was 0.8 [0.2 - 98.4] ng/mL. Using their defined primary outcome of correct localization rate, the authors demonstrated that PyL-PET/CT correctly localized lesions in 84.8-87.0% of cases among the three readers (lower bound of 95% CI: 77.8%-80.4%), against the composite SOT.

The performance of 18F-DCFPyL-PET/CT by CLR (≥1 lesion co-localized) and PPV (≥1 lesion confirmed) was maintained through all 3 SOT categories:
1. Histopathology (N=31): 78.6-82.8% and 92.9-93.3% for CLR and PPV, respectively
2. Correlative imaging (N=100): 86.1-88.6% and 87.0-89.5% for CLR and PPV, respectively
3. PSA response (N=1): 100% for both CLR and PPV
Additionally, CLR remained high regardless of which correlative imaging modality was used including 18F-fluciclovine-PET/CT (N=71; CLR 86.8-90.9%), MRI (N=23; CLR 80.0-86.7%) and CT (n=6; CLR 80.0-100%).
While these imaging characteristics are important, potentially more important is the demonstrated clinical effect of this information. Using local radiographic assessment, PSMA-avid lesion(s) were found in 142 of 208 patients (69.3%). As a result, more than two-thirds of patients enrolled in this study (131 of 205, 63.9%) had a change in intended management following PyL-PET/CT. Of those with a change in management, nearly 80% (103/131, 78.6%) were attributable to positive PyL findings while the remaining 21.4% (28/131) were attributable to negative PyL scans. Changes in management included a transition from salvage local therapy to systemic therapy on the basis of more extensive disease (n=58), a period of observation (n=49), non-curative systemic therapy to attempted curative salvage local therapy (n=43), and observation in place of planned treatment (n=9). In addition to providing this information, PyL was well tolerated with one drug-related SAE (hypersensitivity) and the most common AE being headache (n=4; 1.9%).
Beyond those with biochemically recurrent disease (CONDOR), the 18F-DCFPyL clinical development program includes a variety of other disease spaces which are captured in the OSPREY cohort.27 Patients in both cohort A (high-risk prostate cancer undergoing radical prostatectomy and pelvic lymphadenectomy) and cohort B (suspected recurrent/metastatic prostate cancer based on conventional imaging) were included and underwent 18F-DCFPyL PET-CT performed using a single dose of 9 mCi (333 MBq) of 18F-DCFPyL, administered via intravenous injection and followed by PET/CT acquisition 1 to 2 hours thereafter. 18F-DCFPyL-PET/CT detection rates including lesion counts were systematically analyzed according to TNM staging: prostatic (T), pelvic LN (N), extra-pelvic LN (M1a), bone (M1b), and other visceral organs/soft tissue (M1c). Images were reviewed by three blinded central reviewers and the diagnostic performance of 18F-DCFPyL PET-CT was compared to histopathology.
In cohort A, histologic detection of pelvic nodal disease (with specificity and sensitivity as co-primary endpoints) was defined on the basis of surgical lymphadenectomy. In cohort B, histologic detection was based on biopsy of lesions identified on conventional imaging. In total, 385 patients were enrolled of whom 252 were in cohort A and 93 were in cohort B.
In cohort A, 18F-DCFPyL-PET/CT had median specificity of 97.9% (95% CI: 94.5%-99.4%) and median sensitivity of 40.3% (28.1%-52.5%) among the three blinded reviewers. While specificity met the pre-specified threshold for efficacy, sensitivity did not. The median PPV and NPV were 86.7% (69.7%-95.3%) and 83.2% (78.2%-88.1%), respectively. In cohort B, the median sensitivity and PPV for extra-prostatic lesions were 95.8% (87.8%-99.0%) and 81.9% (73.7%-90.2%), respectively.
As a result of these data from OSPREY and CONDOR, the FDA has given priority review designation to the new drug application for 18F-DCFPyL.
Currently, the limited approval and sparse availability of PSMA-PET/CT in the United States limits its clinical utilization. However, utilization is growing in many other countries and would be expected to follow suit in the United States.
While this work has focused on the use of CT scan as the cross-sectional modality paired with PET imaging, recent work has also assessed the role of PET/MRI, leveraging the advantages of the sensitivity of receptor-targeted imaging and the spatial resolution of MRI.28
In spite of limitations highlighted above and the potential offered by molecularly-targeted imaging, a “conventional imaging” approach utilizing CT scan or magnetic resonance imaging and bone scan remain the mainstay of initial staging as advocated by the most recent NCCN guidelines. The most recent version of the EAU guidelines similarly states that, while staging is warranted for patients with intermediate- and high-risk disease (based on weak and strong recommendations, respectively) and “PSMA PET/CT is more accurate for staging than CT and bone scan”, “to date no outcome data exist to inform subsequent management” (https://uroweb.org/guideline/prostate-cancer/#5) and thus caution must be used when considering the use of these advanced imaging approaches for initial staging in place of conventional imaging.
Conclusion
Ongoing advances in the imaging of prostate cancer have changed our approach to risk stratification and diagnosis. The continuing accrual of data suggests that further advances (some of which have already been published) are likely to change the way we screen for prostate cancer and stage patients following their diagnosis. Understanding the clinical effect of changing imaging modalities is key – there is certainly a risk of the Will Roger’s phenomenon leading to stage migration which may falsely lead us to conclude a clinical benefit from the use of more advanced imaging approaches.
Published Date: May 2021


