Introduction
Bladder cancer is currently the 10th most commonly diagnosed malignancy worldwide. It is estimated that approximately 110,500 men and 70,000 women are annually diagnosed with bladder cancer worldwide.1 While the majority of patients are diagnosed with non-muscle invasive disease (i.e. carcinoma in situ, Ta, and T1), approximately 25 to 33% of patients are initially diagnosed with muscle-invasive bladder cancer and a meaningful proportion of patients initially diagnosed with non-muscle invasive disease will subsequently progress to MIBC.1
Radical cystectomy and trimodal therapy (i.e. maximal TURBT, chemotherapy, and radiotherapy) remain standard of care options for treatment of patients with clinically localized, muscle invasive disease.2,3 However, survival outcomes in this cohort of patients remain limited with five-year survival rates of 70% in patients with bladder-confined disease and 38% in those with extravesical extension and/or lymph node involvement.4
While neoadjuvant cisplatin-based chemotherapy combinations have demonstrated five-year overall survival benefits of at least 5%,5 a significant proportion of patients remain “cisplatin ineligible”, defined by at least one of the following criteria (Galsky criteria):6
- Performance status two or worse
- Glomerular filtration rate <60 ml/min
- Grade >2 audiometric loss
- Peripheral neuropathy
- New York Heart Association class III heart failure
While current guidelines still recommend proceeding directly to radical cystectomy in patients who are cisplatin-ineligible,3 immunotherapeutic agents that have demonstrated efficacy in patients with metastatic bladder cancer7 are increasingly being evaluated in the neoadjuvant setting with promising results. Trials of these agents in this setting have focused on pathologic responses. The recently reported GETUG-AFU V05 VESPER trial reported 42% and 36% pathologic complete responses in patients receiving dose-dense MVAC and gemcitabine/cisplatin, respectively.8 As such, these results provide a benchmark for future neoadjuvant immunotherapy trials in this disease space. In this Center of Excellence article, we review the latest evidence for immunotherapy agent combinations in the neoadjuvant setting prior to radical cystectomy in patients with clinically localized, muscle invasive bladder cancer.
NABUCCO Trial: Ipilimumab + Nivolumab
NABUCCO is a single-arm feasibility trial of 24 patients with stage III urothelial cancer who received two doses each of nivolumab (anti-PD-1) and ipilimumab (anti-CTLA-4), followed by radical cystectomy. Patients were either cisplatin ineligible or had refused cisplatin-based chemotherapy, had a WHO performance status of 0-1, and had received no prior systemic therapy.
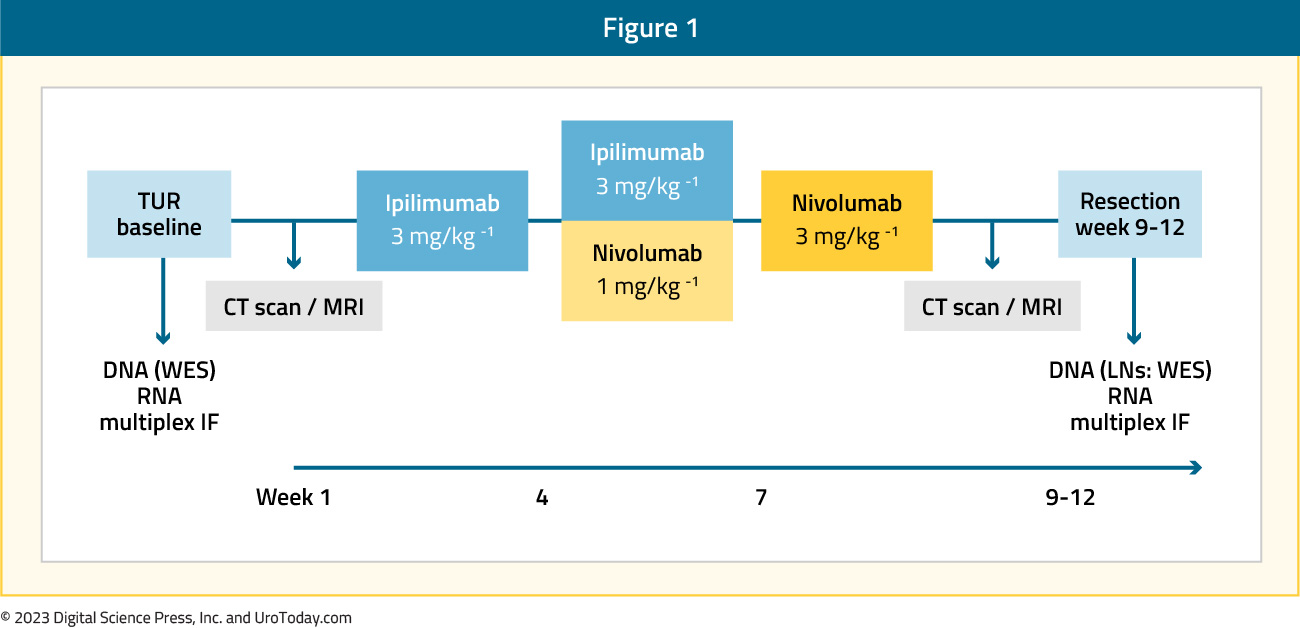
Fourteen (58%) patients had node-negative disease at baseline (cT3-4aN0), and ten (42%) patients had baseline lymph node metastases (cT2-4aN1-3). The primary endpoint was surgical resection within 12 weeks of initiation of neoadjuvant immunotherapy. Twenty-three (96%; 95% CI: 79 to 100%) patients underwent resection within 12 weeks, thus meeting the study’s primary endpoint.
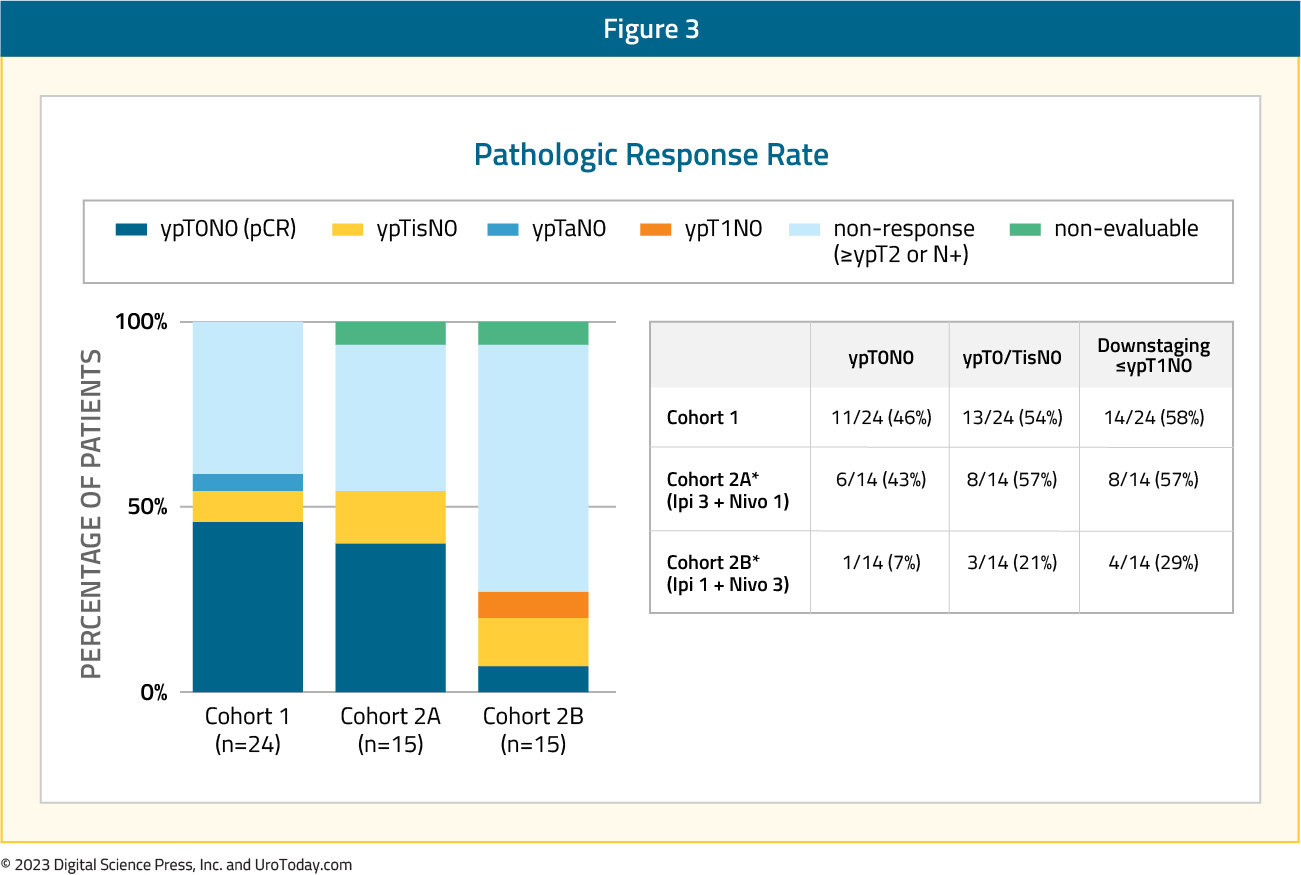
Grade 3–4 immune-related adverse events occurred in 55% of patients and in 41% of patients when excluding clinically insignificant laboratory abnormalities. Eighteen (75%) patients received all three treatment cycles. Six (25%) patients received only two cycles owing to immune related adverse events. There was no treatment-related mortality. A pathologic complete response was observed in 46% of patients, with 58% of patients overall having no remaining invasive disease (pathologic complete response or pTisN0/pTaN0).9
Results of NABUCCO Cohort 2 were presented at ESMO 2021. Pharmacokinetics from cohort 1 revealed that the prior dosing schedule resulted in a short duration of exposure to nivolumab relatively late in the neoadjuvant setting. As such, different doses of ipilimumab and nivolumab were tested as demonstrated below:
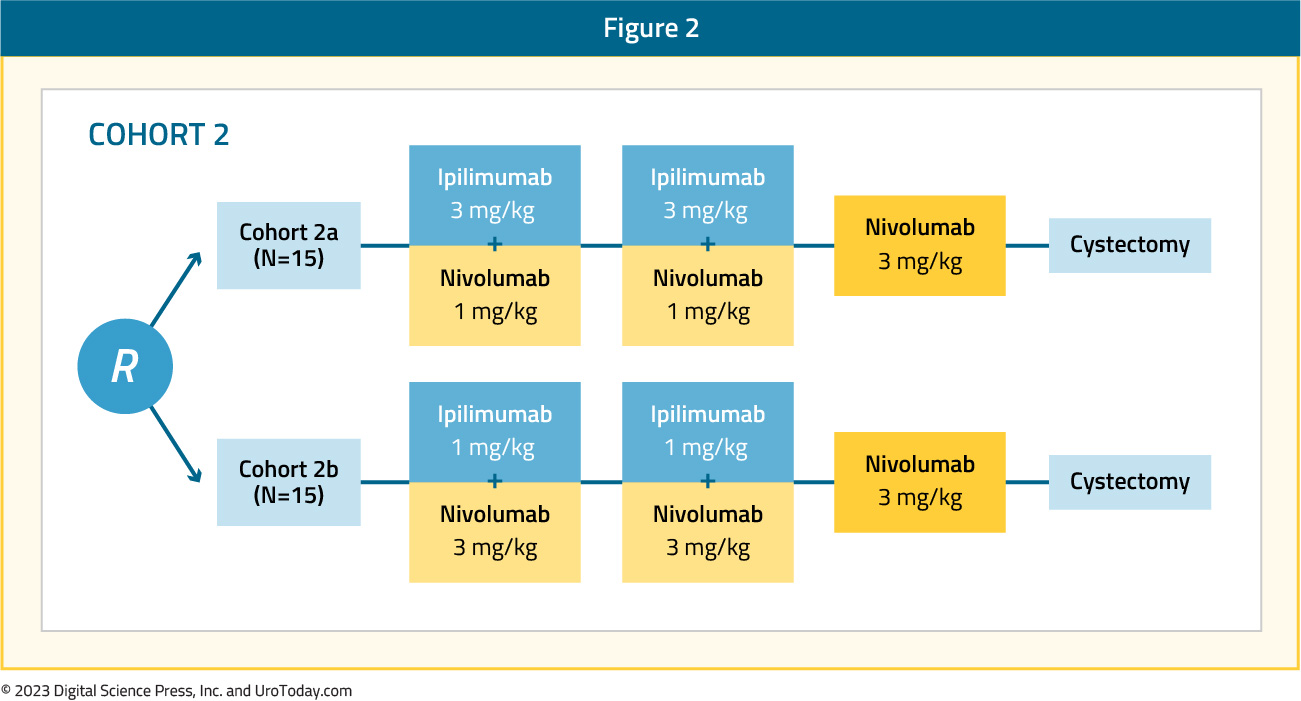
In cohort 2A (ipilimumab 3 mg/kg, nivolumab 1 mg/kg), pathologic complete response was observed in 43% of patients, compared to 46% in Cohort 1. Conversely, pathologic complete responses were noted in only 7% of patients in Cohort 2B (ipilimumab 1 mg/kg, nivolumab 3 mg/kg).
DUTRENEO Trial: Durvalumab + Tremelimumab (Cisplatin Eligible)
The DUTRENEO trial is a randomized phase II trial of durvalumab (anti-PD-L1) and tremelimumab (anti-CTLA-4) versus chemotherapy in urothelial bladder cancer patients prospectively selected by a TIS score. The TIS score is based on an 18-gene IFN-y signaling related expression profile and may be used to select patients who may best respond to immune checkpoint inhibitors.10 The study design is shown below. Of note, all patients were fit for cisplatin and planned for radical cystectomy.

The primary study outcome was pathologic complete response, with secondary outcomes of overall survival, disease-free survival, and toxicity profile. Among patients with “cold tumors”, all of whom received chemotherapy (MVAC, gem/cis, gem/cis/paclitaxel), pathologic complete response was observed in 11/16 (68.8%) patients, with pathologic downstaging in 12/16 (75%). Amongst patients with TIS “hot” tumors:
- Chemotherapy patients (n=22): Pathologic complete response in 36.4% and downstaging in 59.1%
- Durvalumab + tremelimumab (n=23): Pathologic complete response in 34.8% and downstaging in 56.5%
Higher PD-L1 expression levels were associated with improved pathologic complete responses in patients treated with immune checkpoint inhibitors, but not when treated with chemotherapy as demonstrated below. Amongst patients with TIS “hot tumors” treated with durvalumab + tremelimumab, pathologic complete responses were as follows:
- PD-L1 high: 57.1%
- PD-L1 low: 14.3%

NCT02812420: Durvalumab + Tremelimumab (Cisplatin Ineligible)
NCT02812420 is a pilot phase 1 trial that also evaluated the combination of durvalumab and tremelimumab in 28 cisplatin-ineligible patients with high-risk disease features (bulky tumors, variant histology, lymphovascular invasion, hydronephrosis, and/or high-grade upper tract disease). All patients had a baseline TURBT and were subsequently treated with a combination of durvalumab (1500 mg/kg) and tremelimumab (75 mg/kg) every four weeks for a total of two combination doses before cystectomy. cT3 and cT4 disease was present in 43% and 11% of patients, respectively. Twenty-five percent of patients had urothelial carcinoma with variant histology. Grade 3 or worse adverse events occurred in 21% of patients, with elevated lipase (14%), AST (7%), and ALT (7%) levels the most common events. No deaths related to therapy occurred. Twenty-four of twenty-eight (86%) patients completed cystectomy per protocol.
Pathologic complete response, defined as ypT0 or ypTis, was observed in 9/24 patients (37.5%), and the overall downstaging rate (p ≤ T1N0) rate was 58.3% (14 of 24). Notably, in patients with large cT3/4 tumors at baseline, the pathologic complete response and downstaging rates were 42% and 75%, respectively. Results were also encouraging in patients with variant histology (squamous, plasmacytoid, sarcomatoid, micropapillary or small cell), with 4/7 (57%) achieving pathologic complete responses. With a median follow-up of 19.2 months, overall and relapse-free survivals at one year were 88.8% and 82.8%, respectively.11
NCT04610671: Nivolumab + CG0070
Cretostimogene grenadenorepvec (CG0070) is a serotype 5 adenovirus oncolytic vaccine engineered to express GM-CSF and replicate in cells with mutated or deficient RB, with demonstrated efficacy in patients with non-muscle invasive bladder cancer, unresponsive to BCG.12 In this trial, the authors prospectively enrolled cisplatin-ineligible patients with MIBC (cT2-4a, N≤1) to receive CG0070 prior to cystectomy (NCT04610671). Patients received 6 weekly intravesical CG0070 (1x1012vp) instillations and 2 doses of nivolumab at weeks 2 and 6, followed by radical cystectomy.
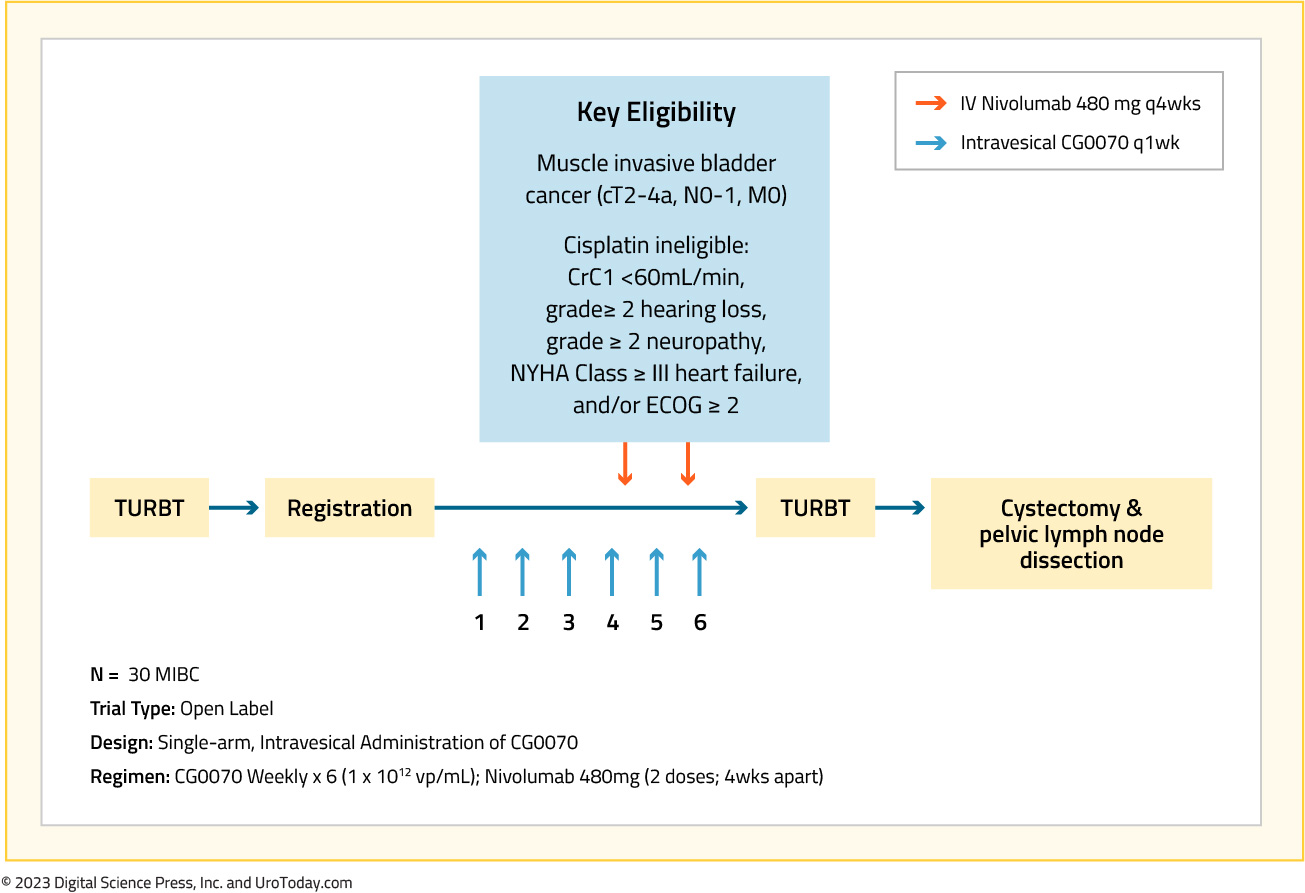
Fifteen cisplatin ineligible patients were enrolled between November 2020 and January 2022. The overall rate of grade 3-4 adverse events was 10/15 (75%), and the vast majority were related to radical cystectomy (90%). Immune related adverse events were seen in one patient, who experienced grade 2 autoimmune thyroiditis. Further, there was no delay in time to radical cystectomy and no unexpected surgical complications from treatment. A complete pathological response was observed in 7/13 (54%) evaluable patients, including the patients who had negative post-treatment biopsy and refused radical cystectomy.
As follows is a table summarizing the outcomes of neoadjuvant immunotherapy combination trials, specifically highlighting ypT0N0 and <ypT2N0 rates: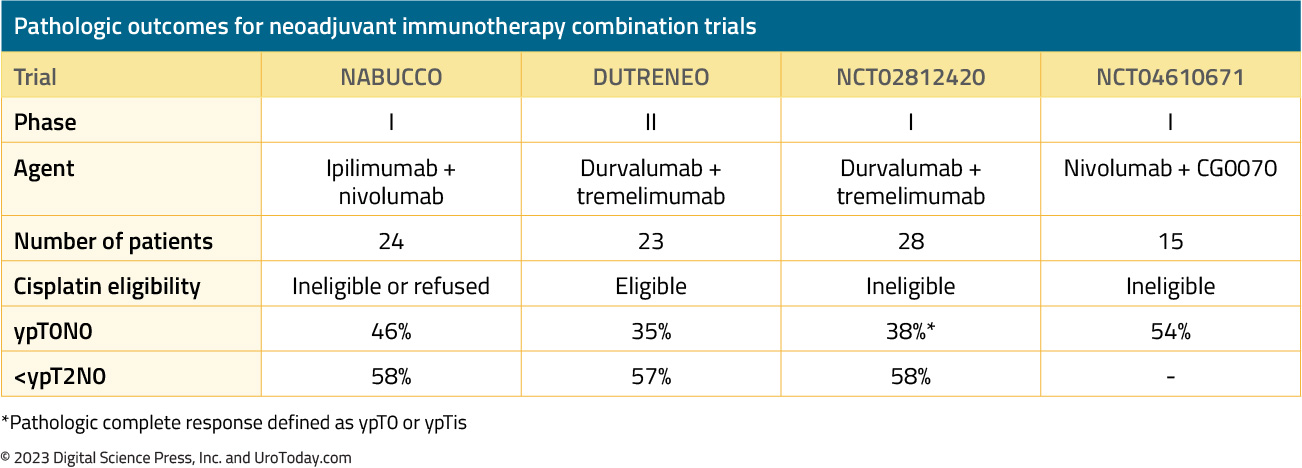
*Pathologic complete response defined as ypT0 or ypTis
Conclusions
Immunotherapy combination trials in the neoadjuvant setting prior to radical cystectomy demonstrate promising early pathologic response rates with complete response rates of 35% to 54%. However, these results are limited to phase I-II trials, and larger scale trials demonstrating a survival benefit are needed before such combinatorial agents become widely adopted in clinical practice.
Published: March 2023


