Imaging in prostate cancer (PC) remains a controversial topic that can be challenging to navigate. In this article, I focus on some of the best tools in our current armamentarium: multiparametric prostate magnetic resonance imaging (mpMRI) for local prostate cancer (PC) and positron emission tomography-computed tomography (PET/CT) for advanced disease. In research settings, these modalities often overlap, but here I take a more practical approach by focusing on the use of PET/CT for the detection of metastatic disease.
Multiparametric Prostate MRI
The American Urological Association (AUA) recommends mpMRI for men whose prostate specific antigen (PSA) level rises following an initial negative standard prostate biopsy.1,2 In the
future, targeted biopsy, using a combination of mpMRI and ultrasound guided transrectal or transperineal biopsy, will likely be the favored method of initial biopsy for patients whose PSA level is elevated or whose digital rectal examination is suspicious for PC.2 It is also likely that mpMRI can benefit men with presumed localized PC before choosing a definitive therapy and to evaluate local recurrence.2
Comparing imaging studies in PC is not easy – they areoften single-site studies and include heterogeneous populations. Nonetheless, a recent meta-analysis of seven studies showed that mpMRI detected PC with a specificity of 88% (95% confidence interval [CI], 82% to 92%) and a sensitivity of 74% (95% CI, 66% to 81%).3 The results held up across subgroups.3
This is reasonably encouraging, but how much is prostatic mpMRI used in practice? In a survey of 302 members of the Society of Urologic Oncology, the Endourological Society, and the European Association of Urology, 86% of respondents reported using prostate MRI and 63% said they used MR-targeted biopsy.4 Urologists were more likely to report using prostate MRI when they practiced in academic settings and performed more than 25 radical prostatectomies a year.4
However, we must keep in mind that response bias is an inherent limitation of such surveys – physicians often do not respond, and respondents tend to be more interested and engaged in the topic than non-respondents.5 Illustrating this point is that in another national survey of 7,400 urologists, only 276 responded.6 Among respondents, users of mpMRI were more likely than nonusers to have completed oncology fellowships, to work in academic centers, and to perform more than 30 prostatectomies annually.6 Respondents also described problems with access, test accuracy, and cost.6 Strikingly, 74% of respondents said that mpMRI “rarely or never” changed their approach to treating intermediate or high-grade PC, while 62% said mpMRI was not helpful for evaluating patients with elevated PSA or an abnormal prostate exam prior to biopsy.6 In addition, only 34% of patients said that their practices used MRI-guided prostate biopsy.6
Results such as these reveal a discrepancy between perceptionand reality in prostatic mpMRI. It is a valuable tool, but work remains to surmount current barriers such as availability, interreader variability, and accuracy.
Availability and Access
Data suggest that mpMRI can be clinically and financially worthwhile if we deploy it for the right patient at the right time. In a recent modeling study of biopsy-naïve men with suspected PC, mpMRI followed by up to two transrectal ultrasound-guided biopsies was cost-effective and detected about 95% of clinically significant PCs.7 The current standard, initial transrectal ultrasound-guided biopsy, had only a 91% sensitivity.7 Also keep in mind that the cost of mpMRI will naturally decrease as it becomes more available. High-quality MRI scanners are plentiful in the United States, including 3T MRI. However, the availability of high-quality prostatic MRI remains limited. Thus, when we talk about lack of availability, we are really talking about availability of quality prostate MRI.
Pirads
We are making strides in terms of quality, especially with the development of the Prostate Imaging Reporting and Data System (PIRADS).8 Sponsored by the American College of Radiology, AdMeTech, and the European Society of Radiology, PIRADS establishes global guidelines for high-quality mpMRI of the prostate. The end goal is to improve detection, localization, characterization, and risk stratification in treatment-naïve men with suspected PC.9
To this end, PIRADS not only defines minimum technical requirements for image acquisition, but also sets standards for communicating the risk and location of aggressive PC. Lexicon is essential and central to radiology – how we communicate is key. Thus, the 64 pages of PIRADS version 2 provides a detailed, standardized algorithm for how radiologists should read prostate MRI based on the location of the abnormality, and a consistent, standardized MR reporting scheme.10
Under the PIRADS system, each prostatic MRI report specifies lesion location, appearance on different sequences, and its PI-RADS score as well as the patient’s most recent PSA level and previous biopsy and MR results. This approach facilitates comparisons between MR reports and biopsy results and also facilitates better longitudinal and multicenter studies. Its complexity makes it clear that prostate MRI should be read by an experienced subspecialized radiologist.
Moreover, quality improvement measures in mammography provided the precedent for PIRADS. In 1994, the United States enacted the Mammography Quality Standards Act and Program (MQSA) to help ensure high-quality breast imaging for women.11 The federally mandated Breast Imaging Reporting and Data System (BIRADS) aimed to reduce inter-operator variability and standardize the lexicon to help make interpretation more decisive and less confusing.12,13
With PIRADS, we’re trying to follow this example to increasequality. To do so, we need to instill the mindset that radiology is not a commodity, and that urologists in both private practice and academia should foster strong working relationships with the radiologists who will be reading these specialized examinations.
Indeed, the AUA guidelines highlight the importance of collaboration and coordination between radiologists and urologists, including the need for radiologists to receive regular feedback about image quality and histopathology results of target lesions mapped on mpMRI, and the need for urologists to receive feedback about quality of the targeted biopsy procedure, including results related to possible image fusion/registration and targeting errors.1 These types of “feedback loops” will help make mpMRI a useful, cost-effective modality in PC clinical care.
Any discussion of diagnostic MRI in 2018 should mention that artificial intelligence (AI) is around the corner and may help improve the quality and throughput of imaging in prostate cancer. Advances in the data sciences will allow computers to use logic, if-then rules, decision trees, and machine learning to mimic human intelligence.17 Machine learning uses complex statistical techniques to improve task performance based on experience, while deep learning facilitates self-training by exposing multilayered neural networks to vast amounts of data.17 With these tools, AI machines in radiology will be able to take abundant data, process it, generate standardized impressions, and self-train and improve over time. This may feel intimidating, but it’s a reality -- and an opportunity we can embrace.
PET/CT for Advanced Disease
In order to effectively manage patients with advanced PC, we need to be able to accurately and precisely quantify the extent of bone and soft tissue disease.17 The imaging of various radiopharmaceuticals using PET/CT is arguably the most valuable tool currently available to cliniciains. Let’s review those most relevant to PC.
C-11 choline
Carbon (C) 11 choline was FDA-approved in 2012 for PET imaging of patients with suspected recurrent PC when bone scintigraphy, CT, or MRI was uninformative.17 C-11 choline PET/CT was later incorporated into the National Comprehensive Cancer Center (NCCN) guidelines as a recommended option for staging patients with biochemical recurrence.18
C-11 choline PET/CT is sensitive for bone and soft tissue disease and can help identify biochemically relapsed patients for referral for salvage therapies.19,20 However, C-11’s short (20-minute) half-life restricts its use to PET centers with an onsite cyclotron and radiopharmacy.17,21
18F-Fluciclovine
Unlike C-11, the radioisotope fluorine (F) 18 has a half-life of 110 minutes.22 Consequently, F-18 tracers can be distributed through networks of existing radiopharmacies, making them more accessible similar to 18F-fluorodeoxyglucose (18F-FDG) which has become the most widely used oncologic tracer.23
In 2016, the FDA approved another F-18 tracer, the synthetic amino acid 18-F-fluciclovine, for PET/CT imaging of patients with biochemical recurrence.24 Robust data support its use: PET/CT with 18-F-fluciclovine has been shown to detect recurrent PC at least as effectively as PET/CT with C-11 choline.25 In January 2018, the NCCN added 18-F fluciclovine PET/CT to its PC guidelines. The test is covered by the Centers for Medicare and Medicaid Services (CMS).18,26
All of this sounds encouraging, but availability of18-F-fluciclovine PET/CT in the community was an early concern as were concerns about the clinical benefits of the exam. The radiopharmaceutical is manufactured by Blue Earth Diagnostics and distributed through PETNET radiopharmacies in 36 U.S. states.24,27 So if access is no longer an issue, the main question becomes the value of the exam. Will it improve outcomes? This is the key question. To answer it, we need to consider lead time bias, the length of time between earlier disease detection and its usual diagnosis. Early diagnosis by screening does not necessarily prolong life, it only lengthens the time a patient lives with a diagnosis. Thus, earlier detection can artificially inflate survival rates, making screening look effective when there is no benefit.28 These are difficult and perhaps impossible questions to truly answer. The one fact we do know is that these exams are more accurate and detect disease much earlier than any of our prior FDA approved diagnostic tools.
Does PET/CT improve management and outcomes?
Next Generation Imaging (NGI) has allowed us to detect recurrent PC earlier. This has been shown to have an impact on management. Ultimately, we want that change in management to improve key outcomes, such as quality of life, progression-free survival (PFS) and overall survival (OS). Understanding limitation bias forces us to ask: does PET/CT for biochemical recurrence meet this standard in PC?
In my opinion, we don’t know yet, but preliminary data are compelling. Recently, in a single-center trial (NCT01666808), 87 post-prostatectomy patients with detectable PSA levels
(biochemical recurrence) were randomly assigned to undergo conventional imaging with or without additional 18-F-fluciclovine PET/CT.29 In a preplanned secondary analysis, positive results of 18-F-fluciclovine PET/CT significantly altered the radiotherapy plan in 41% of cases, a statistically significant result (P < .0001).29 In 75% of cases, radiotherapy field was changed from prostate bed to prostate bed and pelvis. In 25% of cases, the field was narrowed from prostate bed and pelvis to prostate bed.29 Another 6% of patients declined radiotherapy because PET/CT revealed extrapelvic recurrence, but the study was not large enough for this result to be significant (P = .15).29 We await results for the primary outcome of this trial, failure-free survival at 3 years.30
Other studies have examined whether PET/CT alters themanagement of biochemically recurrent PC.31,32 In a recent survey of 126 referring providers, 53% said they made a major change in management after receiving the results of 68-gallium (Ga)- labeled prostate-specific membrane antigen (PSMA)-11 PET.31 Baseline PSA level did not predict whether PET results substantially altered management.31 The researchers recommended
studying whether changes in management ultimately led to better patient outcomes.31
In another single-center study of 68 patients with biochemical recurrence after radical prostatectomy, 60% of patients had recurrence on 18F-DCFBC Prostate-Specific Membrane Antigen-Targeted PET/CT.32 Results varied according to patients’ PSA levels; a PSA level of 0.78 ng/mL predicted a positive PET/CT scan with an area under the curve of .764. Similar to the previous study, positive PET imaging led to changes in treatment strategy for 51% of patients.32
Change in management is a valuable intermediate endpoint– it was the basis for the initial approval of FDG. However, we must acknowledge that the bar is higher now. In most cases, we don’t yet know whether PET/CT ultimately improves outcomes in PC. In the single-arm, prospective FALCON (Fluciclovine (18F) PET/CT in biochemicAL reCurrence Of Prostate cancer) trial (NCT02578940), 18-F-fluciclovine PET/CT substantially altered the clinical management of men with first biochemical recurrence of PC after primary therapy aimed at cure.33 Trial recruitment was halted early after the researchers determined that 61% of patients underwent a change in management post-scan.33 However, data on therapeutic response are still pending.
Summary of imaging options in advanced prostate cancer
What other options do we have besides PET/CT for imaging in advanced PC? Table 1 summarizes these, including their performance for bone and soft tissue imaging and whether they are covered by Medicare.
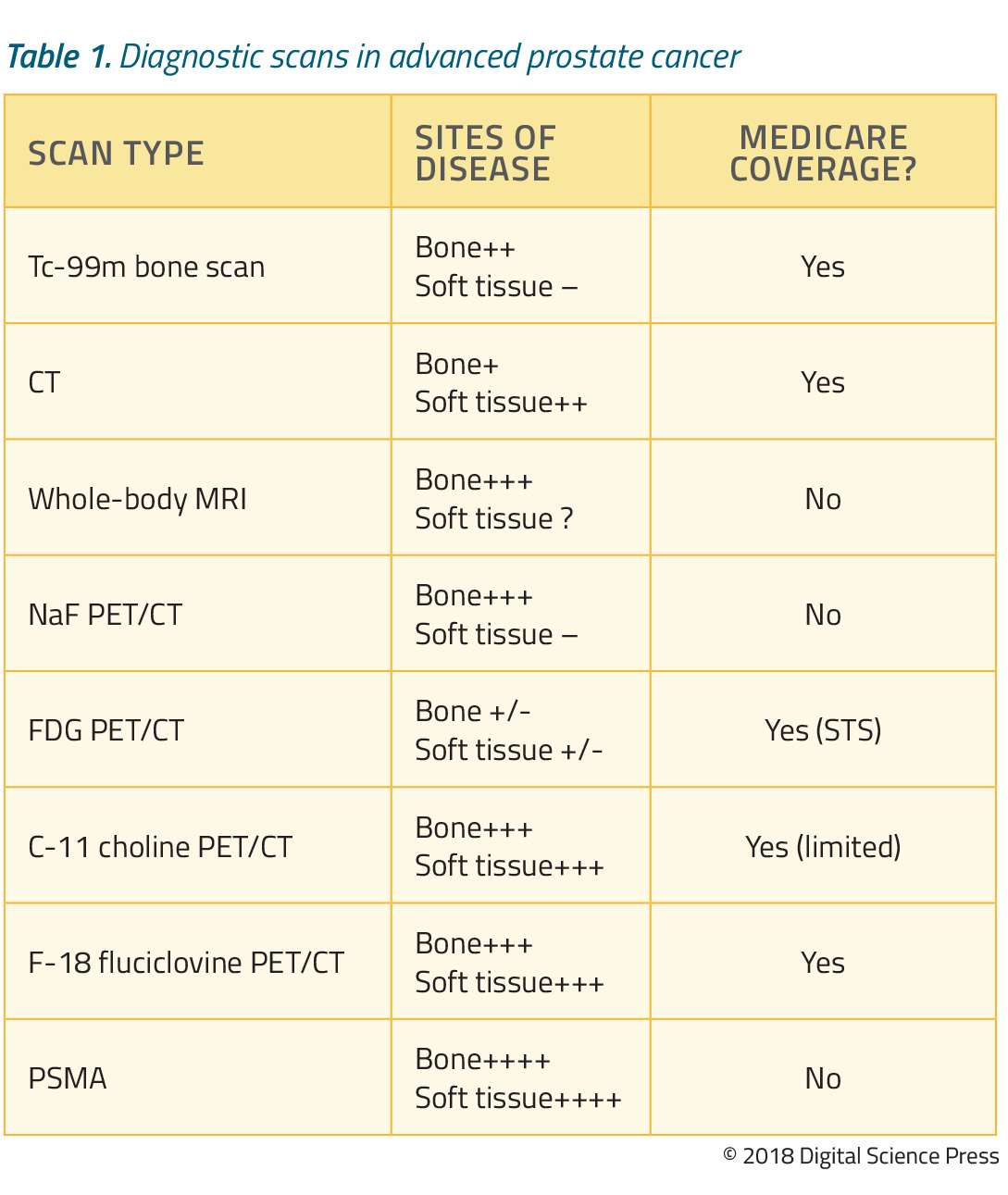
Let’s first consider technetium (Tc)-99m labeled bisphosphonate bone scans. Nearly 90% of patients with metastatic PC have osseous metastases, making this test a mainstay of imaging.34 The Tc-99m scan is highly sensitive for bone metastases and is covered by CMS, but does not reliably detect soft tissue disease.35,36
In contrast to Tc-99-m bone scan, CT performs substantially better for soft tissue, is less sensitive for bone disease, and is also covered by CMS.16,37 Whole-body MRI is excellent for detecting bone disease38 and is commonly used in Europe but there are currently no reimbursable codes in the US.38
Another option is NaF PET/CT, which is excellent for detecting bony disease but less useful for soft tissue disease. Until recently, this test was covered by the National Oncologic PET Registry (NOPR), through which CMS reimbursed PET scans while simultaneously evaluating whether their results influenced patient management.39,40 Unfortunately, this program closed in December 2017 with no plans for renewal. Currently, CMS is evaluating data and we await its decision regarding future coverage.
FDG PET/CT in prostate cancer has not been proven to be beneficial especially at initial diagnosis and biochemical recurrence given the metabolic characteristics of the disease at these specific disease states. However, 18-F-FDG PET/CT has more utility in advanced PC and is covered by Medicare when used to evaluate for “Subsequent Treatment Strategy.”36,41 As discussed, C-11 choline PET/CT provides excellent detection of bone and soft tissue disease; Medicare offers limited coverage.20,36 18-F-fluciclovine PET/CT is highly sensitive for bony and soft tissue disease, and Medicare now reimburses the cost of this test for patients with biochemical PSA recurrence.36
In recent studies, PSMA-based PET imaging was generally superior to all other types of scans for detecting PC bone and soft tissue metastases.42,43,44 In a small head-to-head study of 10 patients with PSA recurrence, five (50%) were negative on 18-F-fluciclovine but were positive on 68-Ga-PSMA.42 Although PSMA-based PET imaging is not yet FDA-approved or reimbursed by Medicare, it’s a tool widely used around the world and will likely be more widely available for use in the US soon.
Theranostics
We cannot discuss imaging in advanced PC without touching on theranostics, an exciting new field that combines targeted therapy with companion diagnostics to advance personalized medicine.45,46 In theranostics, we identify a target on the cell of interest (in PC, it is typically PSMA), attach a binding molecule to the target, and then link this binding molecule to a radioisotope aimed at treatment or diagnosis.46
Clearly, this paradigm is directly applicable to personalized medicine. By using PET/CT with the appropriate tracers, we can identify the distribution and expression levels of a particular receptor and target it.47
Consider a recent study in which PSMA-positive PC tumorswere identified by using Ga-68-PSMA-11 PET/CT and then treated with a beta emitter, (177)Lu-PSMA-617-targeted radionuclide therapy.48 Among 30 patients with metastatic, castration-resistant PC (CRPC) resistant to other treatments, 23 had a PSA response and 13 had more than a 50% decrease in PSA level.48 Among 11 patients who received three treatment cycles, eight had a greater than 50% PSA response for more than 24 weeks, which correlated with radiologic response.48
In this study, patients with bone marrow involvement were more likely to develop treatment-related myelosuppression, but they could be identified ahead of time.48 Based on the findings, the investigators concluded that (177)Lu-PSMA-617 is a “promising new option” for treating mCRPC that needs further study.48
Targeted alpha therapies also deserve our attention. Alpharadiation is short-range and highly cytotoxic.49 The first FDA approved agent was radium 223 which we use to target bone mets in patients with symptomatic mCRPC. This therapy leads to an improvement in overall survival, an outcome not seen with prior nuclear medicine therapies in prostate cancer.50 By attaching an alpha particle to a PSMA targeted agent, we create a much more disease-specific therapy that retains a powerful punch against the disease which is more targeted than traditional beta therapies.
The emergence of targeted alpha therapy creates new possibilities in oncology. In a small cases series of patients with mCRPC, clinicians used Ga-68-PSMA-11 to confirm the presence of PSMA-expressing tumor and then used Ac-225-PSMA-617 as targeted treatment. Two patients thus treated had undetectable PSA levels and complete responses based on restaging with Ga-68-PSMA-11. There were no hematologic toxicities or other treatment-emergent adverse events except xerostomia.51 These treatments are not yet available in the United States, but it’s clear that this is where we’re heading.
Conclusion
Advanced imaging modalities such as mpMRI and PET-CT perform far better than traditional methods for imaging PC. They can detect disease earlier, enabling urologists to change how they manage patients with the goal of improving outcomes. However, reliable data on outcomes are still pending and questions persist about which patients would most benefit from these tools. That being said, we must embrace the improved performance of these tools to find the “sweet spot” where value is maximized.
Projects such as the Prostate Imaging Reporting and Data System (PIRADS) aim to improve standards for imaging and communication, hopefully enhancing the value and cost-effectiveness of mpMRI. We should set similar goals for PET/CT and (in the future) PSMA-targeted imaging and treatment. These tools have tremendous potential if they can be used in the right patients, at the right time, and with careful and accurate communication of findings.
Finally, radiology is not a commodity. It is a highly specialized clinical service where value is optimized when practiced side by side with our urology colleagues. We encourage urologists to give us ready feedback, and work together to push the bar forward with regards to quality and effectiveness of care.
Written By: Phillip J Koo, MD
References
1. Bjurlin MA, Carroll PR, Eggener S, et al. MRI of the prostate, standard operating procedure (SOP) 2017. http://www.auanet.org/guidelines/mri-of-the-prostate-sop Accessed May 9, 2018.
2. Fulgham PF, Rukstalis DB, Turkbey IB, et al. AUA policy statement on the use of multiparametric magnetic resonance imaging in the diagnosis, staging and management of prostate cancer. J Urol. 2017 Oct;198(4):832-838.
3. de Rooij M, Hamoen EH, Fütterer JJ, et al. Accuracy of multiparametric MRI for prostate cancer detection: a meta-analysis. AJR Am J Roentgenol 2014 Feb;202(2):343-351.detection: a meta-analysis. AJR Am J Roentgenol 2014 Feb;202(2):343-351.detection: a meta-analysis. AJR Am J Roentgenol 2014 Feb;202(2):343-351.
4. Muthigi A, Sidana A, George AK, et al. Current beliefs and practice patterns among urologists regarding prostate magnetic resonance imaging and magnetic resonance-targeted biopsy. Urol Oncol 2017 Jan;35(1):32.e1-32.e7.regarding prostate magnetic resonance imaging and magnetic resonance-targeted biopsy. Urol Oncol 2017 Jan;35(1):32.e1-32.e7.
5. Cunningham CT, Quan H, Hemmelgarn B, et al. Exploring physician specialist response rates to webbased surveys. BMC Med Res Methodol. 2015 Apr;15:32.
6. Manley BJ, Brockman JA, Raup VT, Fowler KJ, Andriole GL. Prostate MRI: a national survey of Urologist’s attitudes and perceptions. Int Braz J Urol 2016 May-JUn;42(3):464-471.Urologist’s attitudes and perceptions. Int Braz J Urol 2016 May-JUn;42(3):464-471.
7. Faria R, Soares MO, Spackman E, et al. Optimising the diagnosis of prostate cancer in the era of multiparametric magnetic resonance imaging: a cost-effectiveness analysis based on the Prostate MR Imaging Study (PROMIS). Eur Urol 2018 Jan;73(1):23-30.multiparametric magnetic resonance imaging: a cost-effectiveness analysis based on the Prostate MR Imaging Study (PROMIS). Eur Urol 2018 Jan;73(1):23-30.
8. American College of Radiology. Prostate Imaging Reporting & Data System https://www.acr.org/ Clinical-Resources/Reporting-and-Data-Systems/PI-RADS Accessed June 11, 2018.
9. Turkbey B, Choyke PL. PIRADS 2.0: what is new? Diagn Interv Radiol 2015 Sep; 21(5): 382-384.
10. PI-RADS: Prostatic imaging-reporting and data system. https://www.acr.org/-/media/ACR/Files/ RADS/Pi-RADS/PIRADS-V2.pdf Accessed June 11, 2018.
11. Mammography quality standards act. https://www.fda.gov/downloads/Radiation-EmittingProducts/MammographyQualityStandardsActandProgram/Regulations/UCM110849.pdf
Accessed June 11, 2018.
12. Timmers JM, van Doorne-Nagtegaal HJ, Verbeek AL, et al. A dedicated BI-RADS training programme: effect on the inter-observer variation among screening radiologists. Eur J Radiol 2012 Sep;81(9):2184-2188.
13. Burnside ES et al. The ACR BI-RADS® Experience: Learning From History. J Am Coll Radiol. 2009 Dec; 6(12): 851–860. doi: 10.1016/j.jacr.2009.07.023)
14. Macmillan Dictionary. https://www.macmillandictionary.com/us/dictionary/american/ml_2 Accessed June 11, 2018.
15. Gibbs N, Pine DW, Pollack K. Artificial intelligence: the future of humankind. New York: Time Books, 2017.
16. Padhani AR, Lecouvet FE, Tunariu N, et al. Rationale for modernising imaging in advanced prostate cancer. Eur Urol Focus. 2017 Apr;3(2-3):223-239.
17. Highlights of prescribing information. Choline C11 injection, for intravenous use. https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/203155s000lbl.pdf Accessed June 14, 2018
18. NCCN.org. NCCN Clinical Practice Guidelines in Oncology (NCCN GuidelinesÆ): Prostate Cancer. Version 2.2018- March 8, 2018. https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf Accessed June 14, 2018.
19. Goldstein J, Even-Sapir E, Ben-Haim S, et al. Does choline PET/CT change the management of prostate cancer patients with biochemical failure? Am J Clin Oncol 2017 Jun;40(3):256-259.prostate cancer patients with biochemical failure? Am J Clin Oncol 2017 Jun;40(3):256-259.
20. Evans JD et al. Prostate cancer–specific PET radiotracers: A review on the clinical utility in recurrent disease. Practical Radiation Oncology July 2017 DOI: https://doi.org/10.1016/j.prro.2017.07.011)
21. Ponde DE, Dence CS, Oyama N, et al. 18F-fluoroacetate: a potential acetate analog for prostate tumor imaging--in vivo evaluation of 18F-fluoroacetate versus 11C-acetate. J Nucl Med 2007 Mar;48(3):420-428.
22. Fludeoxyglucose F 18 Injection. Diagnostic: for intravenous only. https://www.accessdata.fda.gov/drugsatfda_docs/label/2005/021870lbl.pdf Accessed June 15, 2018.
23. Wallitt KL, Khan SR, Dubash S, et al. Clinical PET imaging in prostate cancer. Radiographics 2017 Sep-Oct;37(5):1512-1536.Sep-Oct;37(5):1512-1536.
24. FDA-fluciclovine (https://www.fda.gov/newsevents/newsroom/pressannouncements/ucm503920.htm)
25. Nanni C, Schiavina R, Brunocilla E, et al. 18F-fluciclovine PET/CT for the detection of prostate cancer relapse: a comparison to 11C-choline PET/CT. Clin Nucl Med 2015 Aug;40(8):e386-e391.relapse: a comparison to 11C-choline PET/CT. Clin Nucl Med 2015 Aug;40(8):e386-e391.
26. American College of Radiology. HCPCS A9588 Created to Describe Fluciclovine F_18. https://www.acr.org/Advocacy-and-Economics/Coding-Source/ACR-Radiology-Coding-Source-Nov-Dec-2016/HCPCS-A9588-Created-to-Describe-Fluciclovine-F_18 Accessed June 15, 2018.
27. Axumin Imaging Centers. https://www.axumin.com/sites/default/files/2018-06/Imaging-Center-Referral-Site-List.pdf Accessed June 15, 2018.
28. Carlsson SV, Albertsen PC. Better survival after curative treatment for screen-detected prostate cancer compared with clinical diagnosis: a real effect or lead-time bias? Eur Urol 2015 Aug;68(2):183-184; discussion 184-185.
29. Akin-Akintayo OO, Jani AB, Odewole O, et al. Change in salvage radiotherapy management based on guidance with FACBC (fluciclovine) pet/ct in postprostatectomy recurrent prostate cancer. Clin Nucl Med 2017 Jan;42(1):e22-e28.guidance with FACBC (fluciclovine) pet/ct in postprostatectomy recurrent prostate cancer. Clin Nucl Med 2017 Jan;42(1):e22-e28.
30. NIH U.S. National Library of Medicine: Clinicaltrials.gov https://clinicaltrials.gov/ct2/show/NCT01666808 Accessed June 16, 2018.
31. Hope TA, Aggarwal R, Chee B, et al. Impact of 68Ga-PSMA-11 PET on management in patients with biochemically recurrent prostate cancer. J Nucl Med 2017 Dec;58(12):1956-1961.
32. Mena E, Lindenberg ML, Shih JH, et al. Clinical impact of PSMA-based 18F-DCFBC PET/CT imaging in patients with biochemically recurrent prostate cancer after primary local therapy. Eur J Nucl Med Mol Imaging 2018 Jan;45(1):4-11.
33. Teoh EJ, Bottomley DM, Scarsbrook A, et al. The FALCON trial: Impact of 18F-fluciclovine PET/CT on clinical management choices for men with biochemically recurrent prostate cancer. J Clin Oncol 2018;36 (6_suppl):165 (abstr 165)
34. Leung K. 99mTc-2-Methoxyisobutylisonitrile. 2004 Oct 5 Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US);2004-2013.
35. Langsteger W, Haim S, Knauer M, et al. Imaging of bone metastases in prostate cancer: an update. QJ Nucl Med Mol Imaging 2012 Oct;56(5):447-458.J Nucl Med Mol Imaging 2012 Oct;56(5):447-458.
36. Noridian Healthcare Solutions. 2017-2018 Radiopharmaceutical Fee Schedule. https://med.noridianmedicare.com/web/jfb/fees-news/fee-schedules/radiopharmaceutical-fees/radiopharmaceutical-fee-schedule Accessed June 15, 2018.
37. Medicare.gov. Your Medicare coverage: diagnostic tests. https://www.medicare.gov/coverage/diagnostic-tests.html Accessed June 16, 2018.
38. Barchetti F, Stagnitti A, Megna V, et al. Unenhanced whole-body MRI versus PET-CT for the detection of prostate cancer metastases after primary treatment. Eur Rev Med Pharmacol Sci 2016 Sep;20(18):3770-3776.detection of prostate cancer metastases after primary treatment. Eur Rev Med Pharmacol Sci 2016 Sep;20(18):3770-3776.
39. Tunis S, Whicher D. The National Oncologic PET Registry: lessons learned for coverage with evidence development. J Am Coll Radiol 2009 May;6(5):360-365.
40. CMS.gov. Centers for Medicare and Medicaid Services: NaF-18 for bone metastasis https://www.cms.gov/Medicare/Coverage/Coverage-with-Evidence-Development/NaF-18-PET-for-Bone-Metastasis.html Accessed June 17, 2018.
41. Ehman EC, Johnson GB, Villanueva-Meyer JE, et al. PET/MRI: Where might it replace PET/CT? J Magn Reson Imaging 2017 Nov;46(5):1247-1262.
42. Calais J, Fendler WP, Herrmann K, et al. Comparison of 68Ga-PSMA-11 and 18F-fluciclovine PET/CT in a case series of 10 patients with prostate cancer recurrence. J Nucl Med 2018 May;59(5):789-794.in a case series of 10 patients with prostate cancer recurrence. J Nucl Med 2018 May;59(5):789-794.
43. Zacho HD, Nielsen JB, Afshar-Oromieh A, et al. Prospective comparison of 68Ga-PSMA PET/CT, 18F-sodium fluoride PET/CT and diffusion weighted-MRI at for the detection of bone metastases in biochemically recurrent prostate cancer. Eur J Nucl Med Mol Imaging 2018 Jun 6. doi: 10.1007/s00259-018-4058-4. [Epub ahead of print]18F-sodium fluoride PET/CT and diffusion weighted-MRI at for the detection of bone metastases in biochemically recurrent prostate cancer. Eur J Nucl Med Mol Imaging 2018 Jun 6. doi: 10.1007/s00259-018-4058-4. [Epub ahead of print]
44. Alonso O, Dos Santos G, García Fontes M, et al. 68Ga-PSMA and 11C-Choline comparison using a tri-modality PET/CT-MRI (3.0 T) system with a dedicated shuttle. Eur J Hybrid Imaging 2018;2(1):9.tri-modality PET/CT-MRI (3.0 T) system with a dedicated shuttle. Eur J Hybrid Imaging 2018;2(1):9.
45. Jo SD, Ku SH, Won Y-Y, et al. Targeted nanotheranostics for future personalized medicine: recent progress in cancer therapy. Theranostics 2016;6(9): 1362-1377.
46. Yordanova A, Eppard E, Kürpig S, et al. Theranostics in nuclear medicine practice. Onco Targets Ther 2017;10:4821-4828.
47. Kruse V, Belle SV, Cocquyt V. Imaging requirements for personalized medicine: the oncologists point of view. Curr Pharm Des 2014;20(14):2234-2249.
48. Kratochwil C, Giesel FL, Stefanova M, et al. PSMA-targeted radionuclide therapy of metastatic castration-resistant prostate cancer with 177Lu-Labeled PSMA-617. J Nucl Med 2016
Aug;57(8):1170-1176.
49. Nevedomskaya E, Baumgart SJ, Haendler B. Recent advances in prostate cancer treatment and drug discovery. Int J Mol Sci 2018 May; 19(5):1359.discovery. Int J Mol Sci 2018 May; 19(5):1359.
50. Parker C, Nilsson S, Heinrich D, et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 2013 Jul 18;369(3):213-223.
51. Kratochwil C, Bruchertseifer F, Giesel FL, et al. 225Ac-PSMA-617 for PSMA-targeted α-radiation therapy of metastatic castration-resistant prostate cancer. J Nucl Med 2016 Dec;57(12):1941-1944.


