LATITUDE was a Phase III trial of men with high-volume metastatic prostate cancer (PC) that had not been previously treated with androgen deprivation therapy (ADT).1 The patients were randomized to receive standard ADT with placebos vs. ADT plus abiraterone and prednisone. The primary endpoint is overall survival (OS).
After a median follow-up of 30.4 months at a planned interim analysis (after 406 patients had died), the median OS was significantly longer in the abiraterone group than in the placebo group (not reached vs. 34.7 months) (hazard ratio for death, 0.62; 95% confidence interval [CI], 0.51 to 0.76; P < .001). Significantly better outcomes in all secondary endpoints were observed in the abiraterone group, including the time until pain progression, next subsequent therapy for PC, initiation of chemotherapy, and prostate-specific antigen (PSA) progression (P = .009). These findings led to the unanimous recommendation by the independent data and safety monitoring committee that the trial be unblinded and that crossover be allowed for patients in the placebo group to receive abiraterone.
The STAMPEDE study2 also enrolled men with high-volume untreated metastatic hormone-sensitive PC, but was not limited to this group. In fact, only 52% of the patients enrolled in this portion of the STAMPEDE study had metastatic disease; and of that percentage, 88% had bony metastatic disease. The remainder were individuals with locally advanced disease that was either node-positive or 2 or more of the 3 following: Stage T3/4, PSA greater than or equal to 40 ng/mL, and Gleason score of 8 to 10. The headline from STAMPEDE is that, like LATITUDE, a significant OS benefit is apparent in the metastatic group. In the nonmetastatic group, failure-free survival (FFS) was significant, but OS data were not, which is likely the result of an early release of such data in a patient population with a prognosis that is substantially longer than patients presenting with metastatic disease.
Both of these studies were published in The New England Journal of Medicine, and readers are encouraged to read the full articles to gather their own perspective and analysis.
For my own perspective and analysis, read on. I have broken down my analysis as the Five Big Lessons to be learned from LATITUDE’s and STAMPEDE’s abiraterone data.
Number 1: Yet more evidence that earlier intervention is better.
Therapy development in PC has been stuck in chemotherapy quicksand for over a decade. For purely regulatory reasons, the approval of docetaxel in castration-resistant prostate cancer (CRPC) meant that all subsequent treatments had to be developed relative to the centrality of docetaxel. This brings to mind the Latin phrase, In regione caecorum rex est luscus, which means “In the kingdom of the blind, the one-eyed man is king” (Erasmus circa 1500). Thus, as we saw about a decade ago now, the development of enzalutamide, abiraterone, and even radium was dependent on its positioning as a treatment to be administered after docetaxel, or in patients who could not take it, as was the case for radium.
And this created a false sense that newer therapies were developed to somehow allow the use of docetaxel or only be used in the setting of that agent’s failure. In more recent years, however, we‘ve seen the maturation of a series of studies that move novel treatments leftward in the clinical-states model to enable improved outcome, not the delivery of more therapies.
Let’s dissect the abiraterone development strategy, specifically as it informs this discussion. In the COU-301 Study, abiraterone plus prednisone was compared with placebo plus prednisone. Survival was improved by abiraterone, but it was short; however, these were post-docetaxel patients.3 In the COU-302 Study, a modest OS advantage was observed on top of a very significant progression-free survival (PFS) advantage. In COU-302,4 though, patients who started abiraterone therapy on the COU-302 Study with a lower disease burden (based on a PSA in 1 analysis, based on Gleason score, PSA, and degree of symptoms in another),5,6 not only lived longer, as one might expect, but a greater magnitude of benefit was observed over placebo than it was in patients with more advanced disease. Thus, in a way, earlier, low-volume disease is not only better prognostically, but it has predictive significance as well, and the drug worked longer. In COU-301, the agent controlled disease for 6 months, and in COU-302, the disease was controlled for 16.5 months, but in LATITUDE, the malady was controlled longer, albeit in a patient population receiving initial ADT as well. So, COU-302 showed us more definitively that within the broad spectrum of ’‘asymptomatic and minimally symptomatic chemotherapy-naïve CRPC,” earlier abiraterone was better than later.
Furthermore, STAMPEDE, unlike LATITUDE, gives us the opportunity to examine the effect of this hormonal doublet at other points in the disease, most notably patients with high-risk localized and locally advanced disease, who constituted a hefty 48% of the study population. In this group, the ADT plus abiraterone was administered for a finite period of time—2 years, after which the therapy and drug were stopped. Consistent with the long timeline seen in this population, generally, there have been few deaths during the follow-up period. However, a divergence has been noted in FFS. The take-home message here is that early abiraterone conferred an FFS benefit but no OS benefit as yet in patients with T3/4 or N+ or high Gleason disease. The Kaplan-Meier curves in the STAMPEDE Study end at 54 months, but even men with node-positive disease are expected to survive, on average, 10 years or more, so it is no surprise that the OS has not been definitive at this point so far. We can be assured that future years will bring updates on this arm of patients in the STAMPEDE Study.
What does this mean for my practice?
Both the LATITUDE and STAMPEDE Studies support the early use of abiraterone, most certainly in the metastatic disease, ADT-naïve setting. This is an easy conversation. For the locally advanced population, I believe it is also reasonable to have this conversation. It is, after all, a finite period of treatment, and the FFS data from STAMPEDE look fairly impressive. In the M0 cohort of STAMPEDE, the hazard ratio for progression is 0.21, which is an almost 80% reduction in the risk of disease progression with 2 years of abiraterone! So, despite the cost of the abiraterone up-front, it is my guess that by preventing/delaying disease progression to such an extent that it could, in fact, save the cost and morbidity of later ADT and potentially be cost-effective. Also, given the lack of an OS advantage at this point in the follow-up, it appears quite reasonable to have this conversation as well.
If you consider that ADT plus abiraterone for 2 years leads to an 80% reduction in the risk of disease progression, then it follows that this approach will prevent/delay the need for further ADT in a significant proportion of patients who would otherwise experience a serologic relapse (rising PSA without metastasis).
And what about serologic relapse after radical prostatectomy (RP)? In a way, this is the intermediate point between those men with metastases and those with high-risk localized disease. Patients with serologic relapse after RP are one of my most common new visits. Some will die of PC eventually, some will not need any further therapy, and the remainder will live a normal life expectancy and end their lives while receiving treatment for recurrent PC but not die of the disease.
A new trial is currently recruiting patients in the United States, A Study of Androgen Annihilation in High-Risk Biochemically Relapsed Prostate Cancer or AFT-19, which is being conducted through the Alliance for Clinical Trials in Oncology Foundation. This trial is designed to evaluate the efficacy of 1 year of ADT plus abiraterone plus apalutamide and comparing it with ADT alone or ADT plus apalutamide alone.
One hypothesis underlying this trial is that by testing very intense hormonal ablation (actually called “Androgen Annihilation,” even as noted in the title of the protocol) in the setting of minimal disease (rising PSA after RP with negative metastatic disease evaluation) may actually lead to disease eradication in some, or prolonged tumor control in others. It is putting earlier therapy….and more therapy…to the test.
Number 2: Breaking down the benefits into 3 phases may determine how long we use it.
As I think about the implications of the data, I am struck by the question of when the abiraterone benefits are conferred (Figure 1)7 or whether they do so at a continuous rate. Is it in the initial 12 weeks of therapy while the testosterone comes crashing down from the 350–to-600-ng/dL range to the 25-to-50-ng/dL range? This is when the vast majority of the androgen-sensitive prostate cancer cells die, and this is when docetaxel conferred its benefit, as it is only given time 6 times every 3 weeks. Using docetaxel in this early phase of ADT, by definition, means that life-prolonging treatment is really only “in the system” during the time of initial ADT.
The second phase of ”benefit,” theoretically speaking, is in maintaining disease control. For the purposes of this argument, let’s assume that this extends from the point the PSA nadir is reached, likely somewhere between 3 and 7 months, as it has been shown that a high PSA nadir at 7 months is associated with a worse OS.8 I have always wondered what is going on during this time. We know what untreated disease looks like, and we know what CRPC looks like; but what is this entity of controlled, stable metastatic disease?
When I counsel patients about this, I talk about 3 populations of cancer cells. The vast majority are testosterone-addicted and cannot survive without this drug, so they are gone relatively quickly following ADT. A small proportion goes into a senescent state and does not grow. But they don’t die either and are more like hibernating humans on a long space journey in your favorite science fiction movie. Several molecular mechanisms are being studied as participating in this effect.9 Then there are the very rare cells that are so small in number that they don’t become detectable for a long time, but, most importantly, they are entirely unperturbed by ADT and continue to grow. These may be the rare cells that harbor mutations or already have adaptive changes such as androgen-receptor amplification.
Which brings us to part 3 of the abiraterone benefit question: the emergence of CRPC. Does early abiraterone, as given in the LATITUDE and STAMPEDE programs, prevent the emergence of CRPC as its main mechanism of clinical benefit? We know that there are certain characteristics of the CRPC cell that render it sensitive to abiraterone (androgen-receptor amplification and intrinsic androgen production come to mind), but we really don’t know precisely when that begins to happen. If the abiraterone is on board, is that the moment it happens, like nipping the weed in the bud? Is the agent affecting those senescing cells as they wake up from hibernation or does it simply, and only partially, slow the progression of the intrinsically resistant cells?
And this brings us to a clinical decision. For those patients who were destined to respond well to ADT anyway, is this overkill? It’s easy to prescribe abiraterone and prednisone at the initiation of ADT in a man with high-volume disease now. We can most definitely do this, understanding that in the near future we will see a regression of PSA, soft-tissue lesions, and a stabilization of the bone metastasis environment.
But what do we tell these patients 1 or 2 years later when their PSAs are at zero and they feel otherwise, but continue to shoulder high co-pays and have the potential toxicity of daily abiraterone and prednisone? In these cases, perhaps we could either consider intermittent ADT, dropping the abiraterone dose, or potentially discontinuing it altogether. These are the questions that clinical trialists and health authorities alike may wish to consider tackling.
Number 3: A high rate of intrinsic failure still exists.
It is great to see a hazard ratio of survival of 0.63. That’s a continuous and ongoing reduction in the risk of death of 37%, and it is impressive when you think in human terms of what that might lead to: more productive time, more quality time. That’s more time with family and friends and enjoying the parts of life that are most meaningful to us.
But, of course, there is another side to this. A reduction in the risk of death may simply mean that it comes later, so resistance still occurs. Also, and perhaps more importantly, take one look at the Kaplan-Meier curve from the LATITUDE Study and it is readily apparent that about 25% of the patients on the abiraterone arm die within 2 years. That is a surprising, sudden, and very clinically daunting observation.
In the aforementioned Southwest Oncology Group Intermittent vs. continuous ADT study led by Maha H. Hussain, MD, those who did not achieve a PSA nadir of less than 4.0 ng/dL at the 7-month point were not eligible for randomization to continuous vs. intermittent ADT given their perceived poor prognosis. In fact, the median survival of these patients was fewer than 14 months.
What is the biology of this subtype of the disease? I think we could guess fairly accurately. These may be the BRCA2 mutants, the microsatellite instability-high patients and those who harbor other or multiple genomic abnormalities such as PTEN loss, p53 mutation or retinoblastoma loss, all of which have been suggested to be the molecular defects leading to an anaplastic phenotype.10-12Maybe these patients do not need abiraterone, but perhaps they need chemotherapy. It could be that they require something in addition to docetaxel. These are the triple negatives of PC.
Or maybe they are the non-Luminal Bs of prostate cancer. I was privileged recently to be involved in the analysis and publication of research on about 1600 men with PC who had their tumors analyzed by the PAM50 genomic test, which was developed for breast cancer. When the breast cancer genomic model was applied to PC, a very similar outcome pattern emerged to what is seen in breast cancer. Also, one of the classifications, Luminal B, had a very good outcome when patients received postoperative ADT when compared with the other subtype. The answer to this question of intrinsic resistance is in the genomics and in doing a study with the right combination of agents.
We are developing a study for such men at our center and through our PC consortium. In this research, we will study combined taxane and platinum chemotherapy, which will be followed by abiraterone. All patients will undergo the PAM50 classification schema.
Number 4: Acquired abiraterone resistance may not come in the form of rogue, resistant, neuroendocrine, and small cell tumors.
But regardless of these biological considerations in the up-front setting, 1 unavoidable fact is that when CRPC happens, it is worse, not only biologically but also because of the now-reduced number of therapies from which we can choose. Given the disappointing data regarding any efficacy in sequencing enzalutamide after abiraterone in CRPC, it is unlikely that we will see much benefit to the use of enzalutamide as frontline CRPC treatment following the use of abiraterone in frontline hormone-sensitive disease. Thus, when confronting the post-LATITUDE (and therefore abiraterone-resistant) patient, we have a limited number of options available.
It’s at that point in the disease course that we should be doing genomic sequencing (if not yet done) and consider putting in clinical-trial efforts. Fortunately, a number of trials are underway with novel approaches that target DNA repair, the bromodomain, DNA protein kinase, and a variety of other paths, including immunotherapies. Clinical-trial designs will need to, and are, making this adjustment.
We should be rethinking what CRPC is. The use of abiraterone and enzalutamide taught us that we can use hormone therapies after hormonal therapy fails, and we stopped calling it hormone-refractory PC as a result. Maybe we need to go back to that nomenclature because perhaps after abiraterone (and perhaps enzalutamide), the disease really is refractory to hormonal therapy?
Number 5: Those who benefit from chemotherapy may or may not be the same patients who benefit from abiraterone.
In the discussion of the LATITUDE Study at ASCO 2017, Dr. Eric Small presented an overlapping Kaplan-Meier curve of the CHAARTED data, displaying the survival benefit of docetaxel in a very similar patient population (high-volume, hormone-sensitive disease) laid directly on top of the survival curve of the LATITUDE data. The 2 curves are essentially superimposable.
But some have suggested that the form of CRPC that might emerge after more intense ADT with abiraterone plus ADT may induce some sort of more virulent “Frankentumor” that is more aggressive, therefore leading to an earlier death. This does not appear to be the course for many, or even most, of those who started the regimen. But it is possible that some of these patients will go on to develop a small cell or neuroendocrine phenotype.
If this occurs, it would indicate a sudden shift in the risk calculus, indicating that hazard is not something that progresses along some fixed pace. The curve would look a bit weird at that point, but it could happen.
Work at our center and others has focused on the genetics, histology, and clinical outcome of patients with abiraterone-resistant disease. It is a story still being written, and suffice to say we are surprised at this point by the number of cases emerging with neuroendocrine or small cell types. This is occurring in some instances, but not all. These are the cases that may require the earlier integration of chemotherapy.
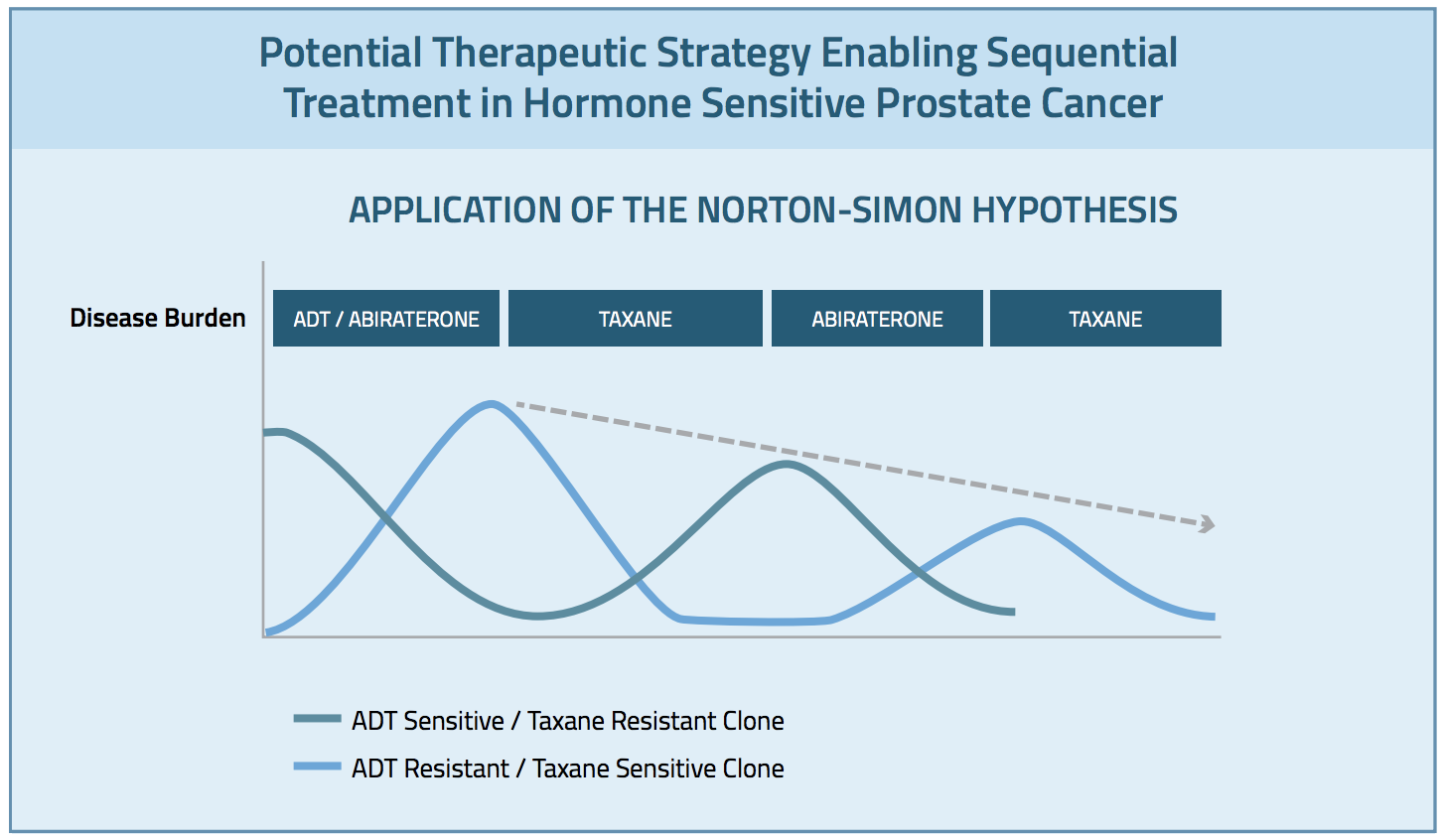
I am reminded of the Norton-Simon hypothesis here.13 This hypothesis centers on the notion that a given tumor may harbor 2 or more subclones that respond to different therapies. Giving the respective therapies in sequence, at full dose, may lead to an enhanced ability to address each of these clones. If this is the case in PC, we need to identify this sequence and study how to go about it optimally. My hunch is that we should not consider the question to be whether it is abiraterone or docetaxel, but whether we should consider a sequence of the two. (Figure 2)7
Figure 1: Theoretical Phases of Response to ADT
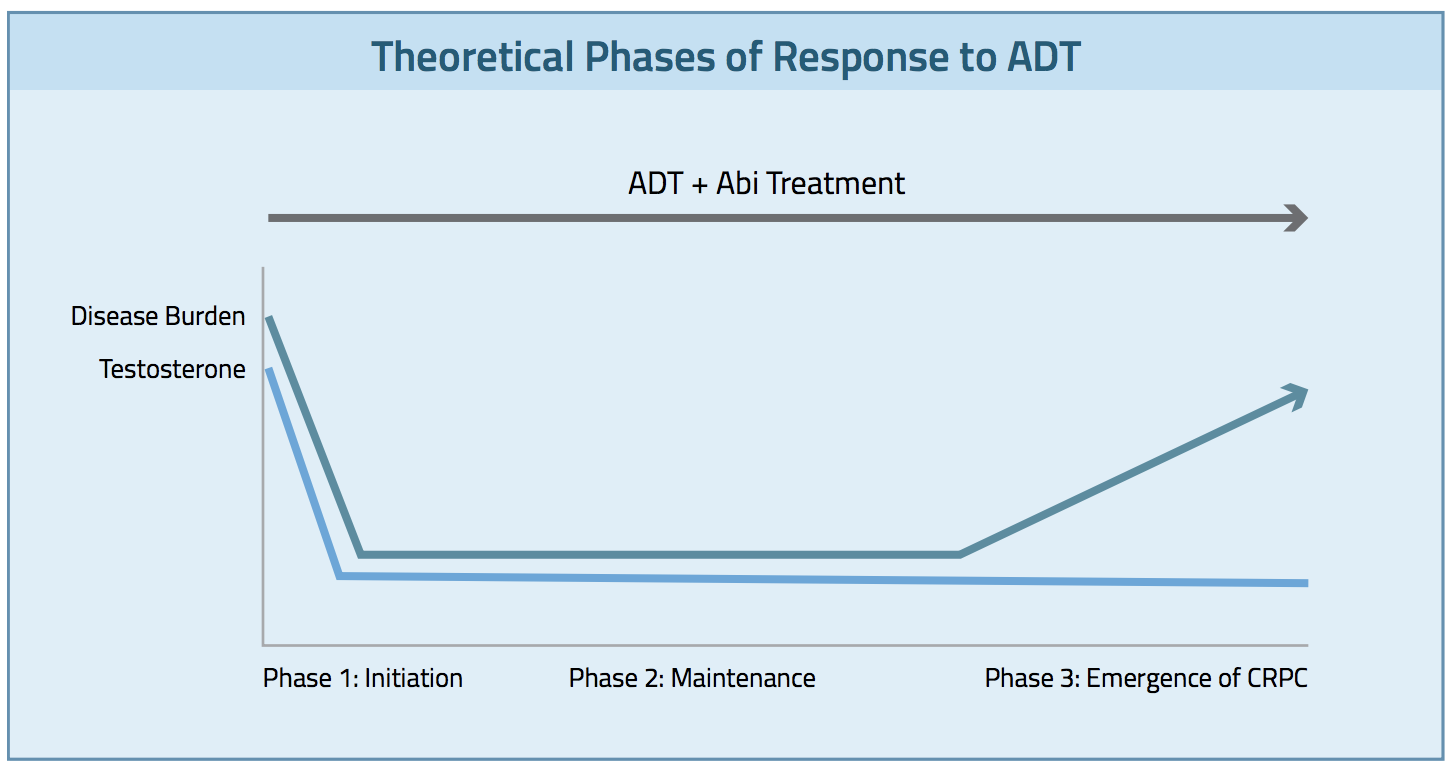
Figure 2: Potential Therapeutic Strategy Enabling Sequential Treatment in Hormone Sensitive Prostate Cancer
Written By: Charles J. Ryan, MD
REFERENCES
1. Fizazi K, Tran N, Fein L, et al. Abiraterone plus prednisone in metastatic, castration-sensitive prostate cancer. N Engl J Med. 2017;377:352-360.
2. James ND, de Bono JS, Spears MR, et al. Abiraterone for prostate cancer not previously treated with hormone therapy. N Engl J Med. 2017;377:338-351.
3. de Bono JS, Logothetis CJ, Molina A, et al. Abiraterone and increased survival in metastatic prostate cancer. N Engl J Med. 2011;364:1995-2005.
4. Ryan CJ, Smith MR, de Bono JS, et al. Abiraterone in metastatic prostate cancer without previous chemotherapy. N Engl J Med. 2013;368:138-148.
5. Ryan CJ, Kheoh T, Li J, et al. Prognostic index model for progression-free survival in chemotherapy-naïve metastatic castration-resistant prostate cancer treated with abiraterone acetate plus prednisone. Clin Genitourin Cancer. 2017 Jul 25. doi: 10.1016/j.clgc.2017.07.014. [Epub ahead of print]
6. Miller K, Carles J, Gschwend JE, et al. The phase 3 COU-AA-302 study of abiraterone acetate plus prednisone in men with chemotherapy-naïve metastatic castration-resistant prostate cancer: stratified analysis based on pain, prostate-specific antigen, and Gleason score. Eur Urol. 2017 Sep 20. doi: 10.1016/jeurouro.207.08.035. [Epub ahead of print]
7. Ryan CJ. The five take-home messages of the LATITUDE and STAMPEDE studies. Everyday Urol Oncol Insights. 2017;2(3):
8. Hussain M, Goldman B, Tangen C, et al. Prostate-specific antigen progression predicts overall survival in patients with metastatic prostate cancer: data from Southwest Oncology Group Trials 9346 (Intergroup Study 0162) and 9916. J Clin Oncol. 2009;27:2450-2456.
9. Esmaeili M, Jennek S, Ludwig S, et al. The tumor suppressor ING1b is a novel corepressor for the androgen receptor and induces cellular senescence in prostate cancer cells. J Mol Cell Biol. 2016;8:207-220.
10. Kudahetti S, Fisher G, Ambroisine L, et al. p53 immunochemistry is an independent prognostic marker for outcome in conservatively treated prostate cancer. BJU Int. 2009;104:20-24.
11. Pourmand G, Ziaee AA, Abedi AR, et al. Role of PTEN gene in progression of prostate cancer. Urol J. 2007;4:95-100.
12. Thangavel C, Boopathi E, Liu Y, et al. RB loss promotes prostate cancer metastasis. Cancer Res. 2017;77:982-995.
13.Simon R, Norton L. The Norton-Simon hypothesis: designing more effective and less toxic chemotherapeutic regimens. Nat Clin Pract Oncol. 2006;3:406-407.


