(Urotoday.com) To kick-off a series of lectures discussing testicular cancer at the inaugural meeting of the Global Society of Rare Genitourinary Tumors (GSRGT) 2020 virtual summit, Dr. Andrea Necchi provided the keynote lecture discussing the management of chemotherapy-resistant germ cell tumors. Dr. Necchi started by highlighting the global incidence and mortality of germ-cell tumors:

As follows is an overview of first- and subsequent salvage treatment options:
- 10-20% of patients will progress/relapse after first-line therapy
- A proportion varying from 25% to 60-70% of patients with will be cured by second-line treatments
- There are very few randomized trials to guide treatment decisions
- Prognosis after second-line therapy is less favorable (5% durable disease-free survival until quite recently)
- Most studies are non-randomized, small and heterogeneous in this patient population
Dr. Necchi notes that there are several conventional-dose salvage therapy options, including single-agent etoposide, single agent ifosfamide, cisplatin + etoposide, cisplatin + etoposide +/- bleomycin, cisplatin + ifosfamide + etoposide, VeIP, and TIP. All studies have relatively small sample sizes with survival rates as low as 7% and as high as 80%: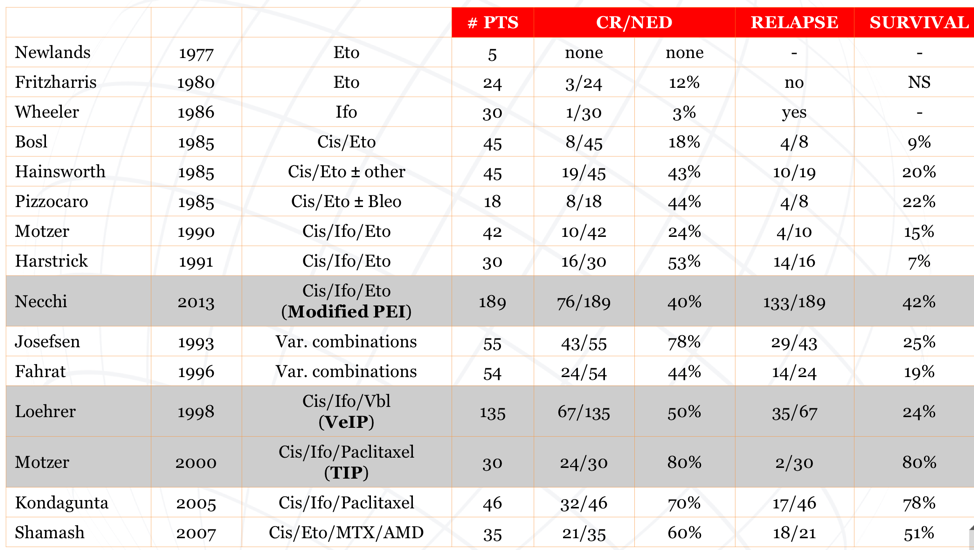
In 2017, the germ cell tumor team at Indiana University published their experience of high-dose chemotherapy and peripheral-blood stem-cell transplantation among patients with relapsed metastatic germ cell tumor.1 There were 364 consecutive patients who progressed after cisplatin-based combination chemotherapy and were subsequently treated with high-dose chemotherapy and peripheral-blood stem-cell transplantation. Three hundred forty-one patients received two consecutive courses of high-dose chemotherapy consisting of carboplatin and etoposide, each for 3 consecutive days, and each followed by peripheral-blood stem-cell transplantation. Over a median follow-up of 3.3 years, the 2-year progression-free survival rate was 60% (95% CI, 55% to 65%) and the 2-year overall survival rate was 66% (95% CI, 60% to 70%). There were 303 patients that received high-dose chemotherapy as second-line therapy with a 2-year progression-free survival rate of 63% (95% CI, 57% to 68%), and 61 patients received high-dose chemotherapy as third-line or later therapy with a 2-year progression-free survival rate of 49% (95% CI, 36% to 61%).
To assess outcomes of conventional versus high-dose chemotherapy at the population level, Lorch and colleagues pooled data from 1,594 patients from 38 centers who progressed after at least 3 cycles of cisplatin-based chemotherapy and treated with either cisplatin-based conventional-dose chemotherapy or high-dose chemotherapy.2 Overall, there were 773 patients who received conventional-dose chemotherapy, and 821 patients who received high-dose chemotherapy. The hazard ratio for overall survival was 0.65 (95% CI, 0.56 to 0.75) favoring high-dose chemotherapy and these results were consistent within each prognostic category except among low-risk patients, for whom similar overall survival was observed between the two chemotherapy-dose groups.
An important ongoing trial spear-headed by the Australian and New Zealand Urogenital and Prostate Cancer Trials Group (ANZUP) collaborative group is the TIGER trial, a randomized trial of TIP versus TI-CE as initial salvage chemotherapy for patients with germ cell tumors. Key inclusion criteria for this trial include (i) histologically confirmed germ cell tumor, (ii) progressive disease after first-line chemotherapy, and (iii) >=3 but <=6 cycles of prior cisplatin-based chemotherapy. The primary outcome for TIGER is overall survival and the trial schema is as follows: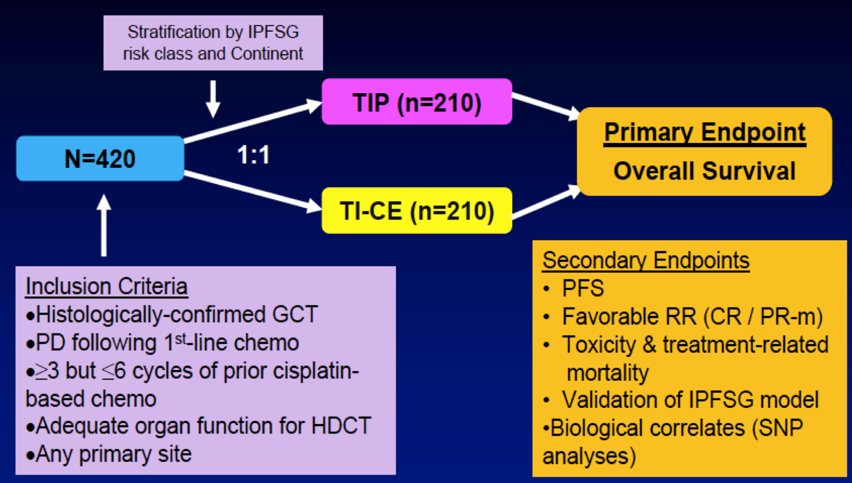
One issue with high-dose chemotherapy salvage treatment is the lack of validated predictors of efficacy. Among studies of high-dose chemotherapy for patients with the poorest outcomes (absolute refractory, third line and further, as well as mediastinal germ cell tumors), the overall survival rates range from 12-64% with ~2 to 5-year follow-up. Despite dramatic improvements in survival of germ cell tumors, mortalities are still devastating with the greatest average number of life years lost (per malignant death). Furthermore, there are no good curative therapies for patients that progress after high-dose chemotherapy. There is also a need to lower the toxicities of current treatment in order to reduce secondary cancers and cardiovascular disease.
Over the remaining slides of his talk, Dr. Necchi discussed early data for several novel treatment regimens for those with refractory germ cell tumors, the majority of which is sobering in regard to poor response rates:
- Single-agent brentuximab vedotin (n=9): ORR of 22.2%, 3-month PFS of 22.2%
- Pembrolizumab (n=12): 0% CR/PR
- Avelumab (n=8): 0% CR/PR
- Durvalumab/tremilimumab (n=11): 9.1% CR/PR
- Cabozantinib/nivolumab/ipilimumab (n=6): 0% ORR
- Nivolumab/ipilimumab (n=4): 0% ORR
- ARQ-197 targeting c-MET (n=27): 19% SD, 74% PD
- Pazopanib (n=43) in the Pazotest trial3: ORR 4.7%, 3 month PFS 12.8%, 12 month OS 14.2%
- Palbociclib targeting cyclin-dependent kinase 4/6: ORR 0%, 52% SD
Dr. Necchi concluded his presentation on the management of chemotherapy-resistant germ cell tumors with the following take-home messages:
- Based on the updated survival estimates, the proportion of patients with advanced germ cell tumors who will ultimately develop the chemoresistant disease is steadily narrowing
- This general improvement in survival is due to an improvement in patient management and the better use of the “old” drugs rather than to the knowledge of tumor biology or the introduction of new effective therapies
- Ensuring the optimal management and access to the ultimate therapeutic strategies or quality surgery is vital for this disease
- Efforts for promoting clinical research to improve outcomes of patients with more aggressive disease will continue to require more international collaboration
- Inclusion of these patients in clinical trials is the most suited approach
Presented by: Andrea Necchi, MD, Associate Professor, Vita-Salute San Raffaele University, Head of Genitourinary Medical Oncology, IRCCS San Raffaele Hospital and Scientific Institute, Milan, Italy
Written by: Zachary Klaassen, MD, MSc, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Augusta, Georgia, Twitter: @zklaassen_md during the 1st Global Society of Rare Genitourinary Tumors Virtual Summit, December 11-12, 2020
References:
1. Adra N, Abonour R, Althouse SK, et al. High-dose chemotherapy and autologous peripheral-blood stem-cell transplantation for relapsed metastatic germ cell tumors: The Indiana University Experience. J Clin Oncol2017 Apr 1;35(10):1096-1102.
2. Lorch A, Bascoul-Mollevi C, Kramar A, et al. Conventional-dose versus high-dose chemotherapy as first salvage treatment in male patients with metastatic germ cell tumors: Evidence from a large international database. J Clin Oncol2011 Jun 1;29(16):2178-2184.
3. Necchi A, Vullo SL, Giannatempo P, et al. Pazopanib in advanced germ cell tumors after chemotherapy failure: Results of the open-bale, single-arm, phase 2 Pazotest trial. Ann Oncol2017 Jun 1;28(6):1346-1351.
Related Content:
GSRGT 2020: Management of Chemotherapy Resistant Germ Cell Tumors in 2020 - Keynote Commentary


