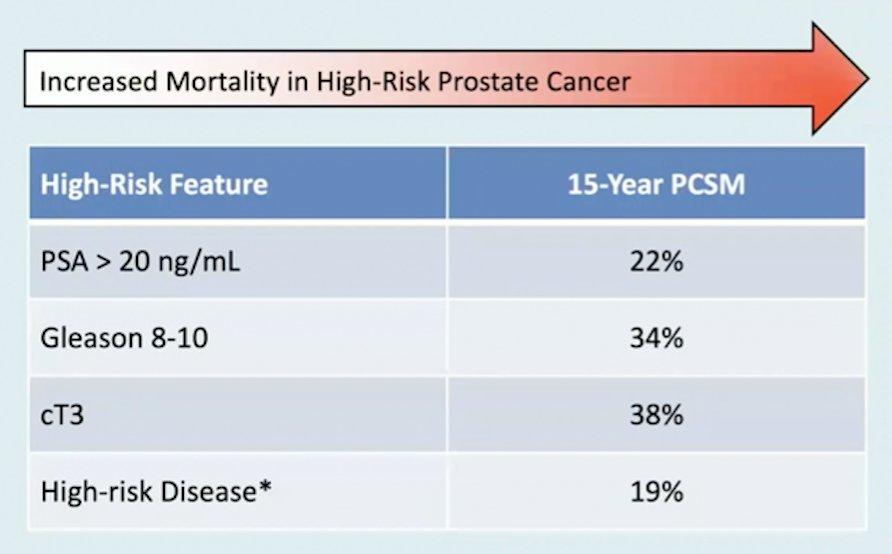
(UroToday.com) The 2022 EAU Section of Oncological Urology (ESOU) Annual Meeting included a session on locally advanced and oligometastatic prostate cancer and a presentation by Dr. Mary-Ellen Taplin discussing neoadjuvant treatments before radical prostatectomy for high-risk prostate cancer. Dr. Taplin notes that ~15% of patients present with localized high-risk prostate cancer, which may be life-threatening to the patient based on 15-year prostate cancer-specific mortality rates as follows:
There are several potential benefits of neoadjuvant treatment for high-risk prostate cancer, which is already standard of care for breast, rectal, and bladder cancer:
- Down-stage local disease, which may facilitate surgical resection
- Reduce or delay post-surgery treatment
- Provide an in vivo assessment of response to treatment
Historical neoadjuvant trials investigated LHRH agonists +/- first-generation anti-androgens, but with the majority of patients having low-risk disease. These trials did not systematically evaluate pathologic response and had limited long-term follow-up. More contemporary neoadjuvant trials are investigating more potent androgen targeting agents (abiraterone acetate, enzalutamide, apalutamide), the majority of patients have high-risk disease, there is systematic central pathology review to evaluate response, and long-term follow-up is ongoing.
The key to the success of neoadjuvant trials is a strong multi-institutional and multi-disciplinary approach, including buy-in from urology, medical oncology, radiation oncology, pathology, biostatistics, radiology, and basic science. As follows is a list of neoadjuvant trials that have been combined with LHRH agonists:
- 2007: Dutasteride versus dutasteride/bicalutamide versus dutasteride/bicalutamide/ketoconazole
- 2009: Abiraterone 3 versus 6 months
- 2012: Enzalutamide monotherapy versus enzalutamide/dutasteride/Lupron
- 2014: Enzalutamide versus enzalutamide/abiraterone
- 2016: Abiraterone versus abiraterone/apalutamide – 6 versus 12 months
- 2019: Phase 3 Proteus trial of Lupron vs Lupron/apalutamide (12 months)
Dr. Taplin asks: What is the impact of intense suppression of the androgen receptor pathway in high-risk prostate cancer? Can we cure more men? As follows is the pathologic complete response and minimal residual disease for key neoadjuvant trials:
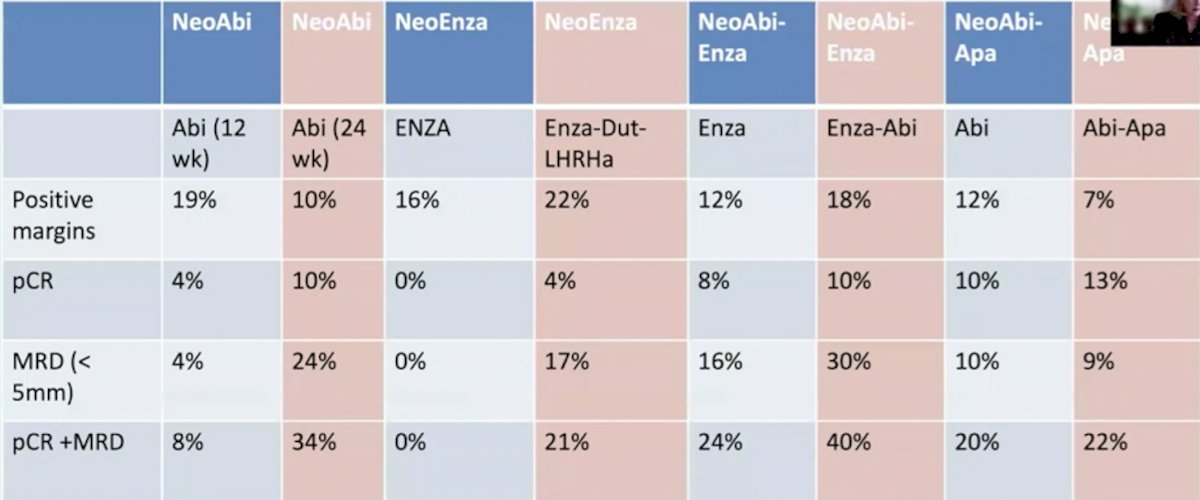
In 2021, McKay et al.1 reported results of 117 post-radical prostatectomy outcomes of patients enrolled in 3 randomized, multicenter, clinical trials of intense neoadjuvant androgen deprivation therapy prior radical prostatectomy. Among these patients, 92 (78.6%) had high-risk disease. Following neoadjuvant therapy, 21.4% (25) had 0-5 mm of residual tumor, including 9.4% (11) with a pathological complete response. Overall, 49 patients (41.9%) experienced biochemical recurrence and the 3-year biochemical recurrence-free rate was 59.1% (95% CI 49.0-67.9). Of the 25 patients with an exceptional pathological response, 2 patients (8.0%) developed biochemical recurrence while 51.1% of nonresponders (47/92) developed biochemical recurrence.

Looking at peri/post-operative complications amongst these patients, estimated blood loss was only a median of 100cc (range 20-100), only 2% of patients had intraoperative complications, and 28-day and 3-month post-radical prostatectomy complications were acceptable, suggesting that high-risk patients that have received neoadjuvant therapy are not at increased risk of complications after radical prostatectomy.
With regards to mechanisms of response and resistance, Dr. Taplin notes that a hypothesis is that ERG+/PTEN loss tumors are larger at baseline and/or have low ADT dependency, suggesting resistance to intense androgen deprivation therapy. Furthermore, tumors with dual ERG+ and PTEN-loss have high residual tumor volume. In 2021, Tewari et al.2 assessed molecular features of exceptional response to neoadjuvant anti-androgen therapy in high-risk localized prostate cancer. In this study, whole-exome and transcriptome sequencing were performed on biopsies from exceptional responders (pathological complete response) and non-responders (pathologic T3 or lymph node-positive disease). Clonal SPOP mutation and SPOPL copy-number loss were exclusively observed in exceptional responders, whereas clonal TP53 mutation and PTEN copy-number loss were exclusively seen in non-responders.
Dr. Taplin concluded her presentation by discussing several important ongoing neoadjuvant trials. First, the Proteus trial is a randomized, double-blind, placebo-controlled, phase 3 study of apalutamide in subjects with high-risk, localized, or locally advanced prostate cancer who are candidates for radical prostatectomy. Patients are randomized to 6 months of apalutamide + ADT versus placebo + ADT followed by radical prostatectomy and 6 months of additional therapy (based on what they received prior to radical prostatectomy) post-operatively. The primary endpoints are pathologic complete response and metastasis free survival, and key secondary endpoints are PSA-free survival and progression free survival. The study schema for Proteus is as follows:

Second, the GUNS trial is a multi-arm, multi-stage adaptive trial in biomarker-selected patients with high-risk localized prostate cancer. The primary endpoint of this trial is pathological complete response rate and the trial schema is as follows:

Third, the Neptune study is a phase 2 multicenter single arm trial (n = 32) of high-risk patients with pathogenic or likely pathogenic germline or somatic BRCA1/2 gene alterations. These patients will be treated with olaparib + LHRH agonist for 6 months followed by radical prostatectomy. The primary endpoint for this trial is pathologic complete response + minimal residual disease, with the following trial schema:

Finally, neoadjuvant darolutamide + abemaciclib will be assessed in a phase I lead-in 3+3 design to determine RP2D, followed by a randomized phase 2 study of ADT + darolutamide + abemaciclib versus ADT + darolutamide followed by radical prostatectomy. The primary endpoint is rate of pathologic complete response + minimal residual disease rate, with the following trial schema:

Dr. Taplin concluded her presentation of neoadjuvant treatments before radical prostatectomy for high-risk prostate cancer with the following summary points:
- Currently, there is no phase 3 data to support neoadjuvant systemic therapy with radical prostatectomy
- Phase 2 data shows promise for ADT with second-generation agents, including favorable pathologic complete response, minimal residual disease, PSA relapse, and salvage therapy rates
- The phase 3 Proteus trial will complete accrual of 2,000 participants in May 2022, with a co-primary endpoint of pathologic complete response/minimal residual disease and radiographic progression-free survival. It will also be important if this trial can validate pathological complete response rate as a surrogate for radiographic progression-free survival
- Biomarkers suggest favorable response in SPOP mutated and poor response in p53 mutated and ERG+/PTEN-loss
- There are ongoing correlative analyses to investigate response and resistance, including single-cell sequencing
- Future trials are investigating novel strategies, including novel combinations (PARP inhibitors, immunotherapy, targeted therapy), biomarker stratification, and PSMA-PET as a biomarker of response
Ultimately, the goal is increased cure rates for men with localized high-risk prostate cancer.
Presented by: Mary-Ellen Taplin, MD, Harvard Medical School, Dana-Farber Cancer Institute, Boston, MA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 EAU Section of Oncological Urology (ESOU) Hybrid Annual Meeting, Madrid, Spain, Fri, Jan 21 – Sun, Jan 23, 2022.
References:
- McKay RR, Berchuck J, Kwak L, et al. Outcomes of Post-Neoadjuvant Intense Hormone Therapy and Surgery for High Risk Localized Prostate Cancer: Results of a Pooled Analysis of Contemporary Clinical Trials. J Urol. 2021 Jun;205(6):1689-1697.
- TewariAK, Cheung ATM, Crowdis J, et al. Molecular features of exceptional response to neoadjuvant anti-androgen therapy in high-risk localized prostate cancer. Cell Reports. 2021;36:109665.


