(UroToday.com) The 2022 EAU Section of Oncological Urology (ESOU) Annual Meeting included a session on urothelial cancer and a presentation by Dr. Valeria Panebianco discussing how magnetic resonance imaging (MRI) has changed the bladder cancer pathway. Dr. Panebianco notes that for more than 30 years the diagnostic pathways for bladder cancer patients have remained largely unchanged, with TURBT as the initial diagnostic and staging tool. However, there are TURBT-related issues, such as understaging and residual tumor (~58%), which are often secondary to operator-dependent performance (experience, judgement, skill).
The rationale and aim of VI-RADS was to define a standardized approach to imaging and reporting mpMRI for bladder cancer, defining the risk of muscle invasion.1 Furthermore, VI-RADS was created through a consensus using existing literature. The scoring is applicable to untreated patients and to treated patients having only received a diagnostic TURBT, but prior to re-TURBT. mpMRI is best performed before or at least 2 weeks after TURBT, bladder biopsy, or intravesical treatment. Administration of an intramuscular antispasmodic agent is recommended, in addition to adequate bladder distention. MRI does not necessarily have the ability to visualize all of the histological bladder wall layers, however, it is able to assess size, location, multiplicity, and morphology. A 5-point VI-RADS score is generated using the individual T2W, DWI, and DCE MRI categories and suggests the probability of muscle invasion. The dominant sequences for risk estimates are DWI (first) and DCE (second, especially if DWI is sub-optimal). The T2 sequence (structural category) is helpful as a first pass guide.
The VI-RADS scoring is as follows:
- VI-RADS 1: SC CE and DW category 1 (muscle invasion is highly unlikely)
- VI-RADS 2: SC, CE and DW category 2; both CE and DW category 2 with SC category 3 (muscle invasion is unlikely to be present)
- VI-RADS 3: SC, CE, and DW category 3; SC category 3, CE or DW category 3, and the remaining sequence category 2 (the presence of muscle invasion is equivocal)
- VI-RADS 4: At least SC and/or DW and CE category 4; the remaining category 3 or 4 SC category 3 plus DW and/or CE category 4; SC category 5 plus DW and/or CE category 4 (muscle invasion is likely)
- VI-RADS 5: at least SC plus DW and/or CE category 5; the remaining category 4 or 5 (invasion of muscle and beyond the bladder is very likely)
There are clinical implications in different settings for the utilization of VI-RADS, as highlighted in the following figure:
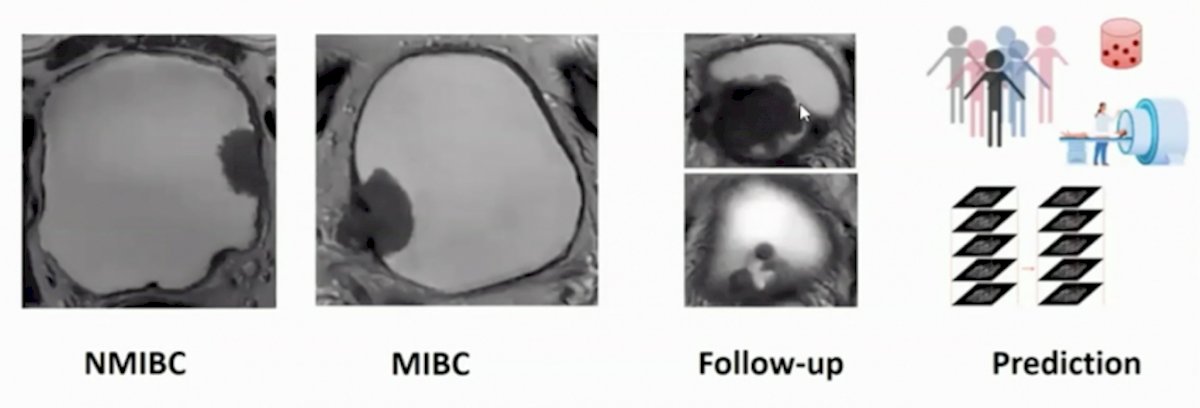
Dr. Panebianco’s group published a study prospectively validating VI-RADS for discrimination between NMIBC and MIBC at TURBT, and evaluated the accuracy of VI-RADS for identifying high-risk NMIBC patients who could avoid re-TURBT and detecting those at higher risk for understaging after TURBT.2 There were 231 patients with bladder cancer suspicion that were offered mpMRI before TURBT, and according to VI-RADS, a cutoff of ≥3 to define MIBC was assumed. mpMRI showed sensitivity, specificity, PPV, and NPV for discriminating NMIBC from MIBC at initial TURBT of 91.9% (95% CI 82.2-97.3), 91.1% (95% CI 85.8-94.9), 77.5% (95% CI 65.8-86.7), and 97.1% (95% CI 93.3-99.1), respectively. Furthermore, the AUC was 0.94 (95% CI 0.91-0.97). Among HR-NMIBC patients (n=114), mpMRI before TURBT showed sensitivity, specificity, PPV, and NPV of 85% (95% CI 62.1-96.8), 93.6% (95% CI 86.6-97.6), 74.5% (95% CI 52.4-90.1), and 96.6% (95% CI 90.5-99.3) respectively, to identify patients with MIBC at re-TURBT (AUC 0.93, 95% CI 0.87-0.97).
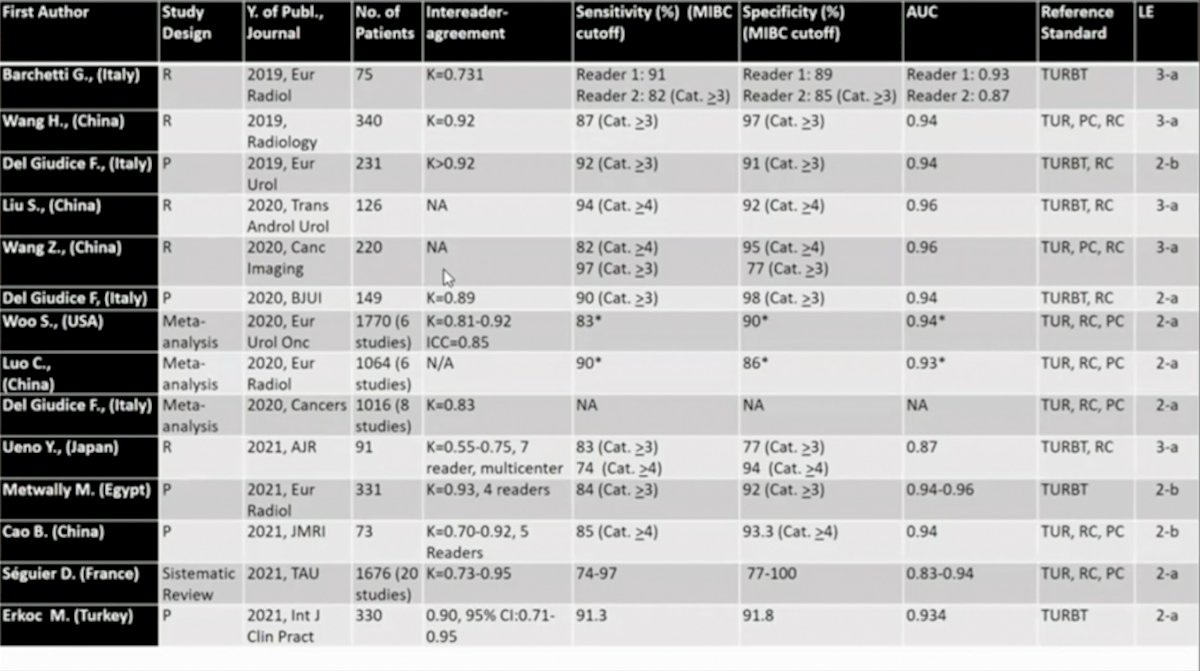
In 2020, Woo and colleagues performed a systematic review and meta-analysis3 to review the diagnostic performance of VI-RADS for the prediction of MIBC among six studies and 1,770 patients. For this study, the pooled sensitivity and specificity were 0.83 (95% CI 0.70-0.90) and 0.90 (95% CI 0.83-0.95), and the area under the curve was 0.94 (95% CI 0.91-0.95). Meta-regression analyses showed that the number of patients (>205 vs ≤205), magnetic field strength (3 vs 1.5 T), T2-weighted image slice thickness (3 vs 4 mm), and VI-RADS cutoff score (≥3 vs ≥4) were significant factors affecting heterogeneity (p ≤ 0.03). In a more recent meta-analysis (December 2021) assessing VI-RADS accuracy, Jazayeri et al.4 included 22 eligible studies, consisting of 2,576 participants and 5,414 MRI reports. The area under curve of VI-RADS at cut-point values of 3 and 4 were 0.93 (95%CI: 0.91, 0.95), 0.93 (95%CI: 0.90, 0.95), respectively. The optimal VI-RADS cutoff value for predicting MIBC was determined as 3 which granted a pooled sensitivity of 89% (95%CI: 87%, 91%; I2=48%) and a specificity of 84% (95%CI: 80%, 87%; I2=90%). Based on meta-regression, the sources of inter-study heterogeneity for VI-RADS ≥ 3 were the sample size > 70, study design, single-center vs multi-center, patient population characteristics (i.e., gender, age), reference standard, histology, magnetic strength, T2WI slice thickness, and the number of radiologists reporting the MRI results.
Dr. Panebianco notes that since their initial publication in 2018, there has been several studies that have provided (retrospective) validation of the scoring system, with a high inter-reader agreement:
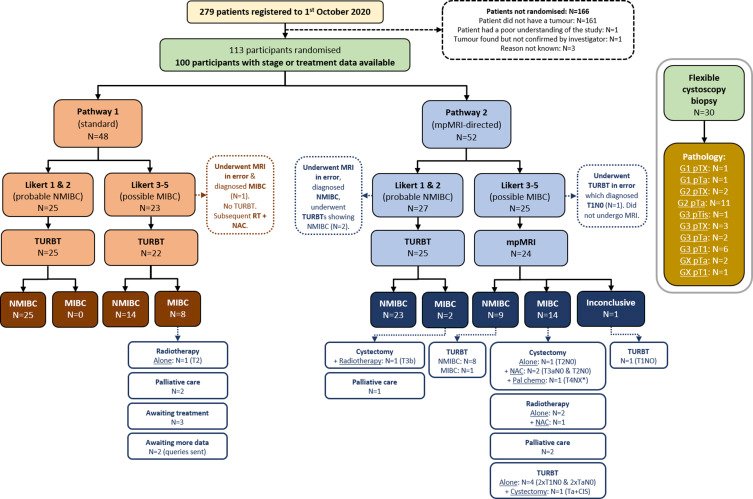
Initial results of the BladderPath study were recently published in 2021,5 randomizing patients to risk-stratified (5-point Likert scale) image-directed care with TURBT for patients with newly diagnosed bladder cancer. In this initial assessment, 279 patients were screened and 113 were randomized, with reporting on the first 100 participants to complete staging: 48 in pathway 1 (TURBT) and 52 in pathway 2 (mpMRI for possible MIBC, Likert 3-5). Fifty of 52 participants designated Likert 1-2 (probable NMIBC) from both pathways were confirmed as having NMIBC (96%). Ten of 11 cases diagnosed as NMIBC by mpMRI have been pathologically confirmed as NMIBC, and 10/15 cases diagnosed as MIBC by mpMRI have been treated as MIBC (5 participants underwent TURBT):
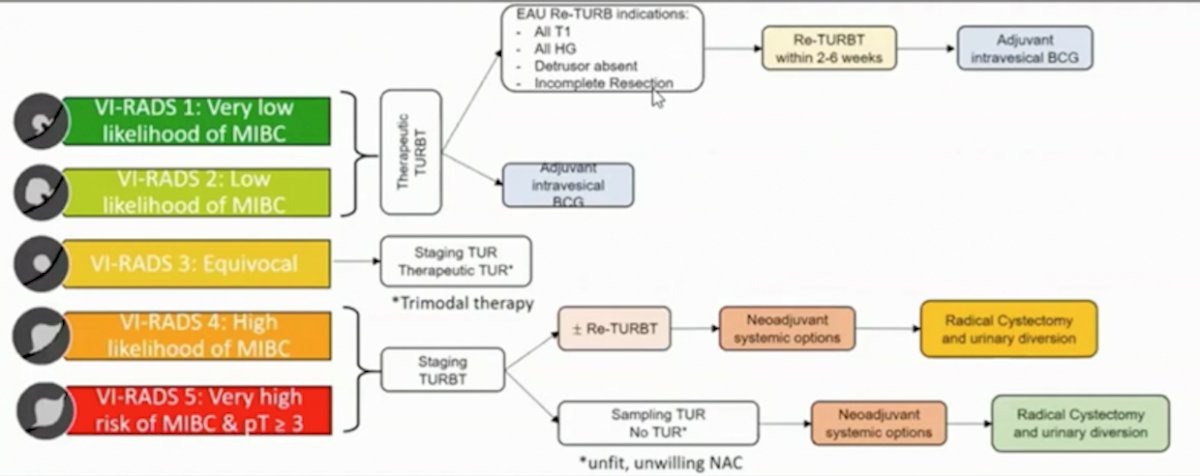
The proposed MRI pathway for patient stratification prior to definitive treatment is as follows:
Recently, Cao and colleagues assessed the application of VI-RADS in the post-treatment setting.6 Among 73 patients (n=42 with primary bladder cancer, n=31 with post-treatment bladder cancer), there was no difference between the AUCs in the primary and post-treatment groups (p = 0.870), with a cut-off for the post-treatment group of ≥4. Preliminary work has also assessed the utilization of VI-RADS for assessing response to systemic therapy for bladder cancer.7 In this study, 10 consecutive patients diagnosed with non-metastatic MIBC were prospectively enrolled to receive neoadjuvant chemotherapy, as well as an mpMRI before staging resection and after the chemotherapy cycles. NacVI-RADS categories were able to match all the final radical cystectomy pathology both for complete pT0 responders and for the patients defined as partial or minimal responders, who only showed some radiologic assessment of response inter-scoring class downstaging.
Dr. Panebianco concluded her presentation of VI-RADS and MRI for bladder cancer management with the following take-home messages:
- VI-RADS can assess risk of muscle invasion
- VI-RADS can be used to lower the burden of unnecessary TURBT procedures
- An MRI pathway should enhance patient satisfaction
- MRI has shown promising results for assessment of cancer response to neoadjuvant chemotherapy or immunotherapy
Presented by: Valeria Panebianco, Professor, Sapienza Universita de Roma, Rome, Italy
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2022 EAU Section of Oncological Urology (ESOU) Hybrid Annual Meeting, Madrid, Spain, Fri, Jan 21 – Sun, Jan 23, 2022.
References:
- Panebianco V, Narumi Y, Altun E, et al. Multiparametric Magnetic Resonance Imaging for Bladder Cancer: Development of VI-RADS (Vesical Imaging-Reporting and Data System). Eur Urol 2018 Sep;74(3):294-306.
- Del Giudice F, Barchetti G, De Berardinis E, et al. Prospective assessment of Vesical Imaging Reporting and Data System (VI-RADS) and its Clinical Impact on the Management of High-risk non-muscle-invasive bladder cancer patients candidate for repeated transurethral resection. Eur Urol 2020 Jan;77(1):101-109.
- Woo S, Panebianco V, Narumi Y, et al. Diagnostic performance of Vesical Imaging Reporting and Data System for the Prediction of Muscle-invasive Bladder Cancer: A Systematic Review and Meta-analysis. Eur Urol Oncol. 2020 Jun;3(3):306-315.
- Jazayeri SB, Dehghanbanadaki H, Hosseini M, et al. Diagnostic accuracy of vesical imaging-reporting and data system (VI-RADS) in suspected muscle-invasive bladder cancer: A systematic review and diagnostic meta-analysis. Urol Oncol. 2021 Dec 9;S1078-1439(21)00504-4.
- Bryan RT, Liu W, Pirrie SJ et al. Comparing an Imaging-guided Pathway with the Standard Pathway for Staging Muscle-invasive Bladder Cancer: Preliminary Data from the BladderPath Study. Eur Urol. 2021 Jul;80(1):12-15.
- Cao B, Li Q, Xu P, et al. Preliminary Exploration of the Application of Vesical Imaging-Reporting and Data System (VI-RADS) in Post-Treatment Patients with Bladder Cancer: A Prospective Single-Center Study. J Magn Reson Imaging 2022 Jan;55(1):275-286.
- Pecoraro M, Del Giudice F, Magliocca P, et al. Vesical Imaging-Reporting and Data System (VI-RADS) for Assessment of Response to Systemic Therapy for Bladder Cancer: Preliminary Report. Abdom Radiol (NY). 2021 Dec 17 [Epub ahead of print].


