(UroToday.com) The 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023 was host to a non-prostate genitourinary proffered paper session. Dr. Lisa Pickering delivered the discussant session for the preceding three oral abstract presentations:
- LBA87 - Phase 2 LITESPARK-003 Study of Belzutifan in Combination With Cabozantinib for Advanced Clear Cell Renal Cell Carcinoma (ccRCC)
- LBA88 - Belzutifan versus everolimus in participants (pts) with previously treated advanced clear cell renal cell carcinoma (ccRCC): randomized open-label phase 3 LITESPARK-005 study
- 1881O - Safety and Efficacy of Two Doses of Belzutifan in Patients (pts) With Advanced RCC: Results of the Randomized Phase 2 LITESPARK-013 Study
Dr. Pickering began by highlighting important qualitative data from RCC advocacy groups that clearly demonstrate that although complete responses remain their priority, we must not forget about other aspects that remain important to these patients, including treatment durability, improved quality of life, and reduction of symptoms. New treatment regimens in this space have delivered improved outcomes, with durable responses/cures in some cases. However, in most cases, the treatments are of time-limited benefit, and, as such, there is an ongoing need for new therapeutic strategies.

As has been previously highlighted in the preceding presentation, belzutifan is a Hypoxia-inducible Factor (HIF-2a) inhibitor that blocks downstream oncogenic pathways. Constitutively active HIF-2a is a key driver in von Hippel Lindau disease and clear cell RCC. Belzutifan works within the cancer cell and is not a ‘multi-targeted’ tyrosine kinase inhibitor (TKI). As such, it may have the greatest activity in cancers most dependent on VHL, HIF, and VEGF.
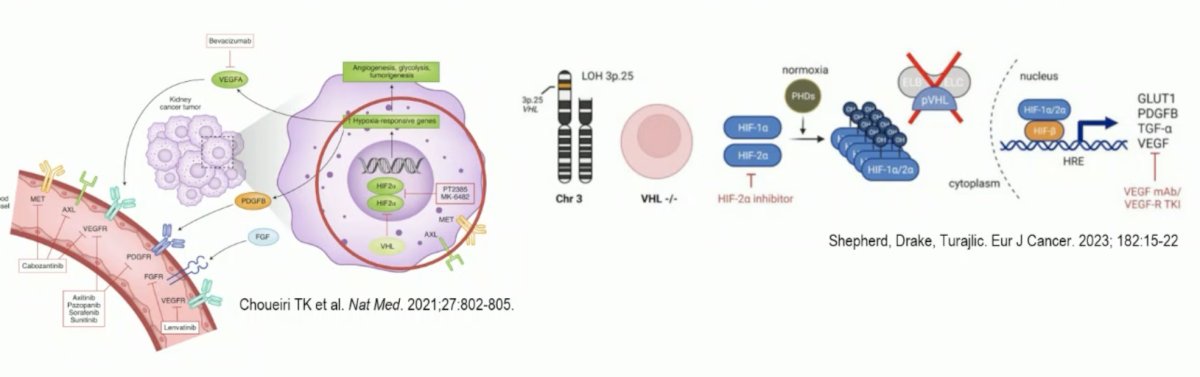
Dr. Pickering noted that previous work has demonstrated that belzutifan has activity in VHL disease and VHL-associated RCC. In the New England Journal of Medicine publication by Jonasch et al. in 2021, it was demonstrated that an ORR of 49% was observed in 61 belzutifan treated RCC patients. Additionally, there was an observed response for pancreatic neuroendocrine tumors (77%) and retinal hemangiomas in 16/16 eyes among 12 patients.1
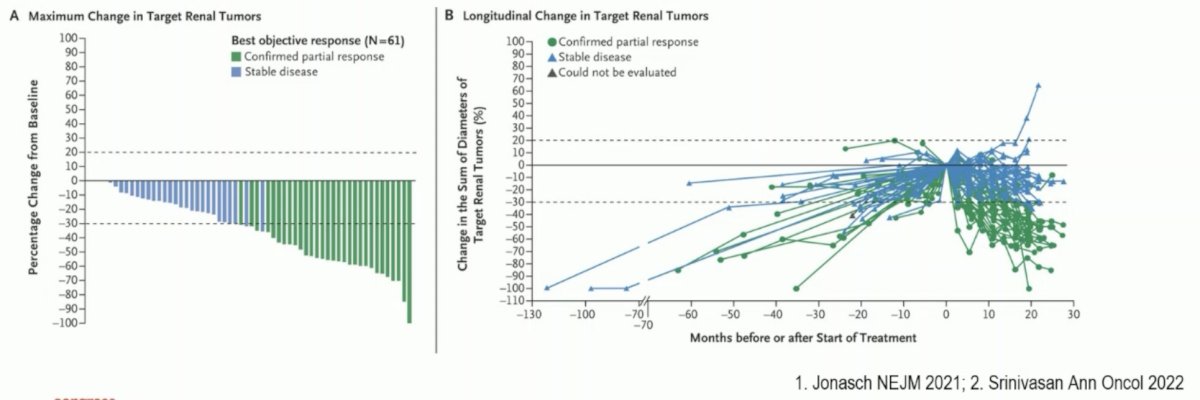
The LITESPARK-001 phase 1 dose-escalation study in patients with advanced solid tumors and dose expansion in those with previously treated advanced clear cell RCC demonstrated that no dose-limiting toxicities occurred at doses up to 160 mg once daily, and the maximum tolerated dose was not reached. The recommended phase 2 dose was 120 mg once daily. In the dose expansion cohort receiving 120 mg orally once daily, the median PFS was 14.5 months, with an ORR of 25%.2
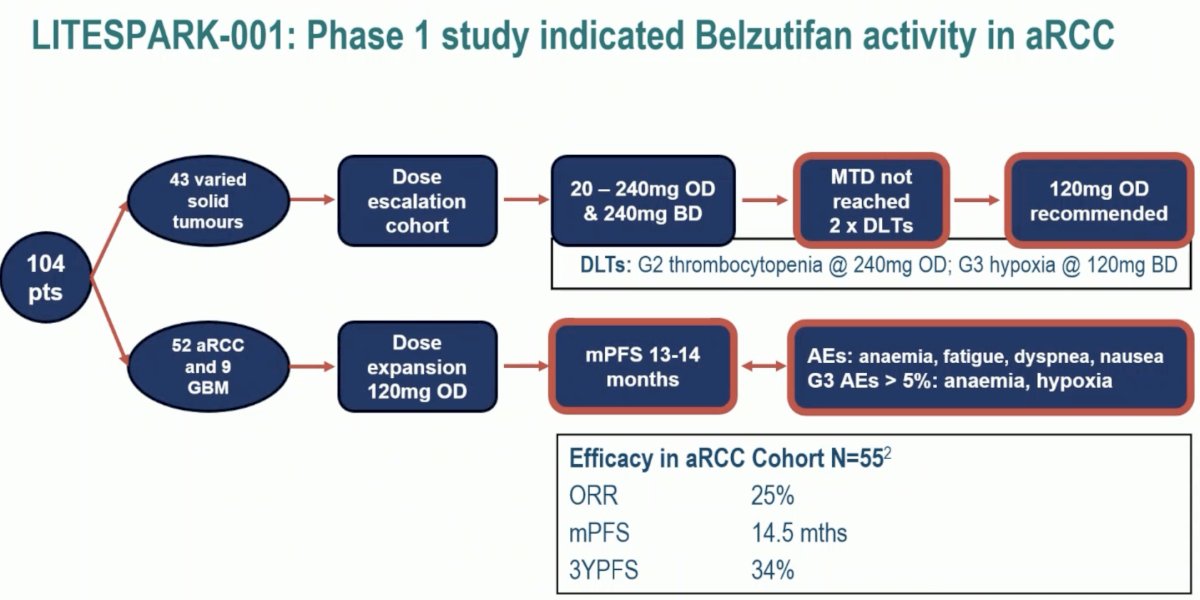
As such, these studies provided a strong foundation for the continued evaluation of belzutifan 120 mg in the advanced ccRCC disease space.
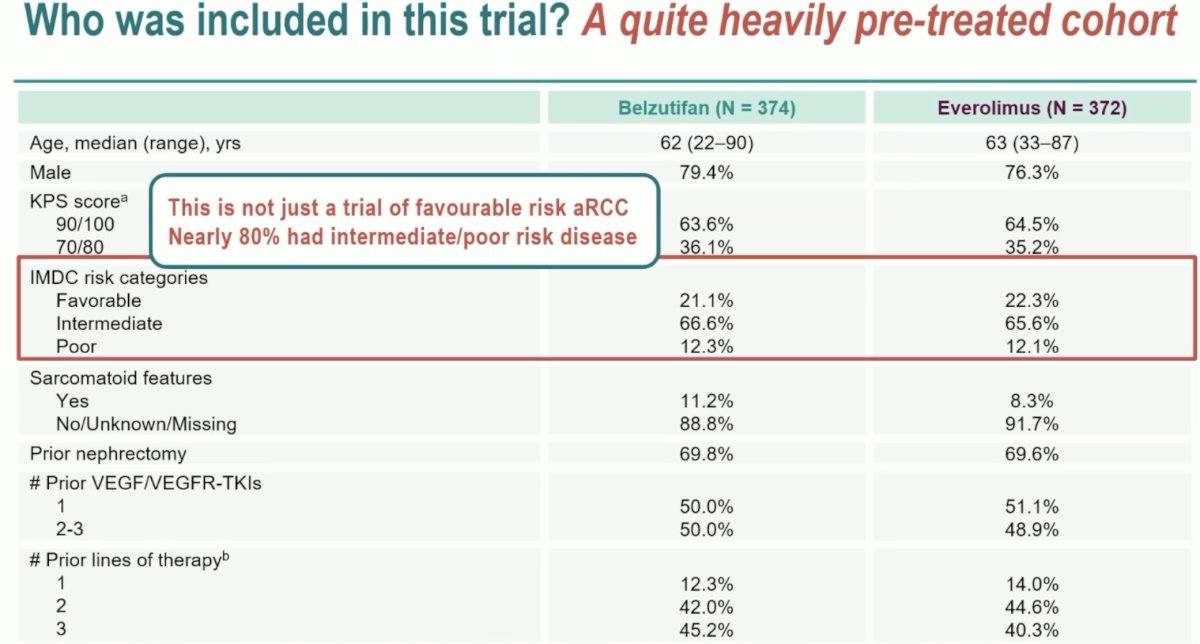
Of the three prior presentations, Dr. Pickering began by discussing the phase 3 LITESPARK-005 study. She highlighted that this trial included a heavily pre-treated cohort of patients with all having received a prior IO and TKI agent, 50% had 2 or 3 prior TKIs, and >85% were treated in the 3rd or 4th line setting in this trial. In such a heavily pre-treated cohort, it is important to consider the possibility of further acquired somatic mutations that may have important implications for treatment selection and response to biological agents. She noted that 80% of patients had intermediate/poor risk disease, which is important given that patients with worse IMDC risk scores may have a better response to belzutifan compared to the favorable risk subgroup.
This trial met its PFS co-primary endpoint with a HR of 0.74 (95% CI: 0.63 – 0.88). Significantly, the HR remained stable across the interim analysis time points, providing further assurances that this is likely a ‘real’ effect.
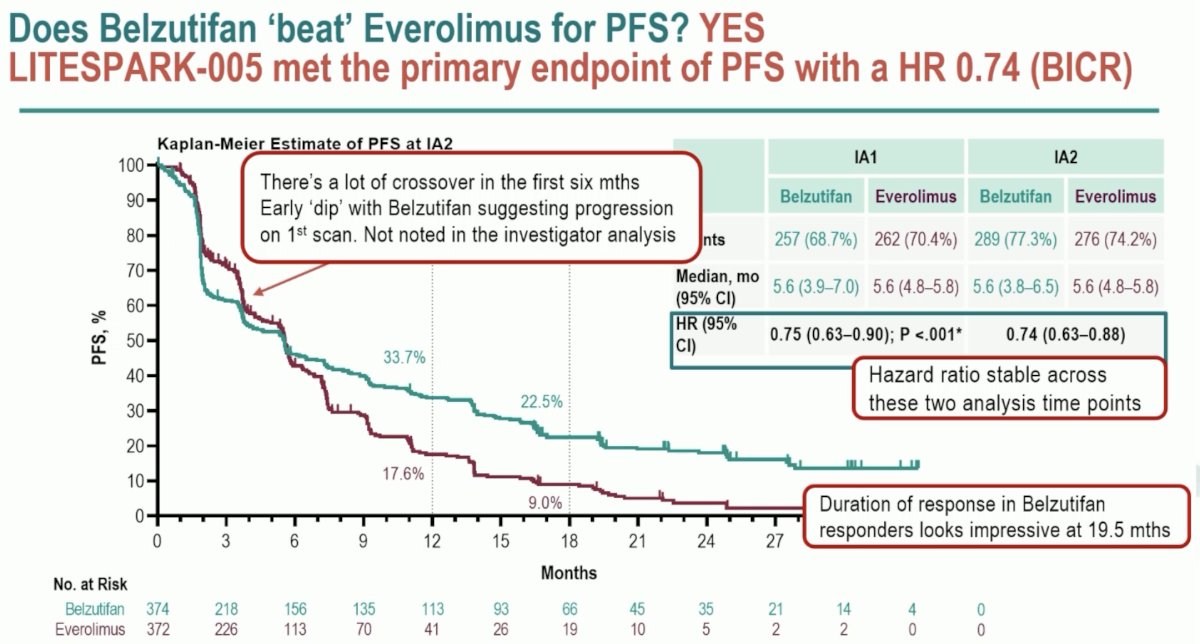
There are however some important key takeaways from the Kaplan Meier curve above. There was a crossover of the curves within the initial 6 months, which may reflect a ‘dip’ with belzutifan suggesting progression on the 1st scan on BICR. Additionally, the duration of response in Belzutifan responder appears to be impressive at 19.5 months.
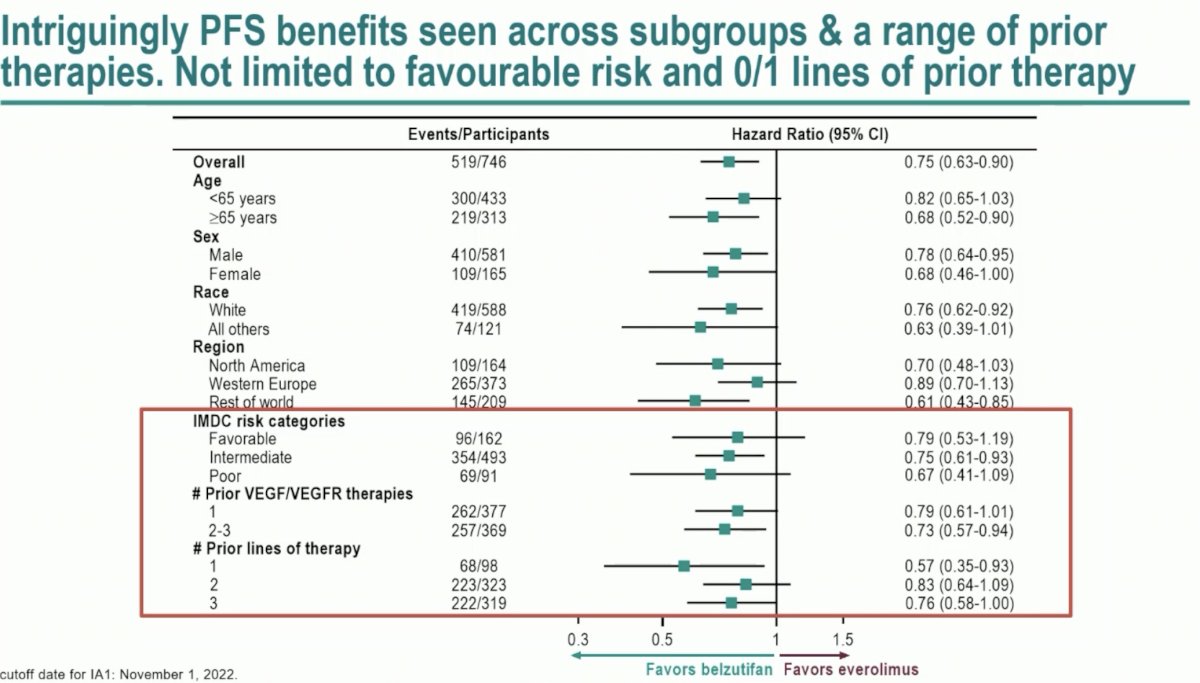
Significantly, PFS benefits were observed across subgroups and a range of prior therapies. This benefit is not limited to those with IMDC favorable risk disease and 0-1 prior lines of therapy.
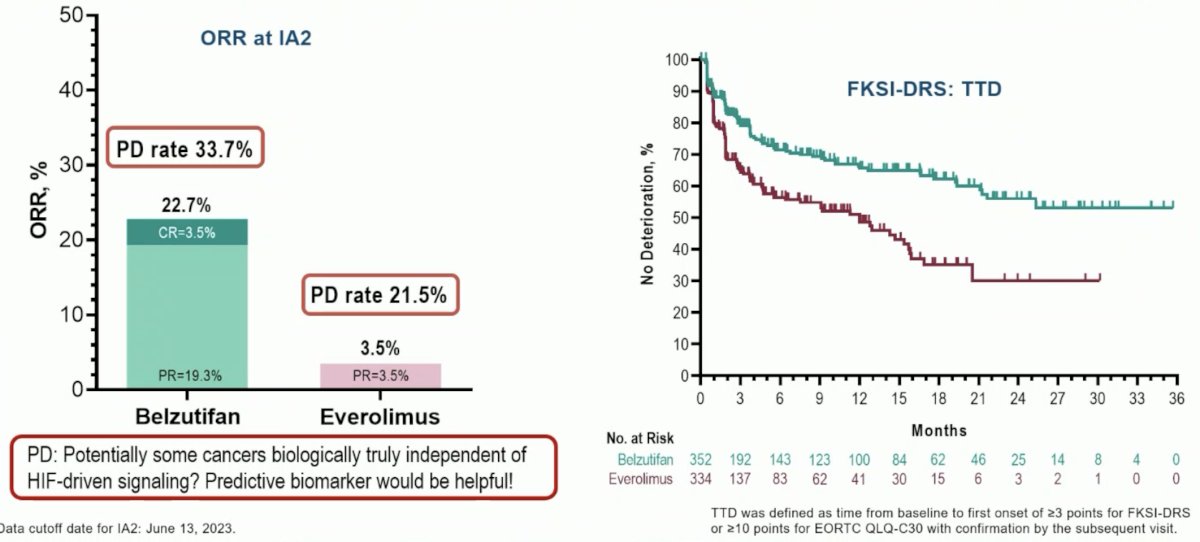
Other key takeaways from this study include an ORR of 23%, although the primary progressive disease rate was also high at 34%. From a patient quality of life standpoint, belzutifan was well-tolerated in the study and the quality-of-life curves separated early.
To date, no OS benefits have been observed with belzutifan, compared to placebo, in this setting. While OS data remains immature, 441 of the 483 required events have occurred. Additionally, the HR for OS has remained stable at 0.87-0.88 across the evaluable study time points, suggesting that we are unlikely to observe an OS benefit in the future.

Dr. Pickering next reflected on the choice of the control arm everolimus:
- Was everolimus the optimal choice? She argued that at the time of the study design it certainly was, although nowadays, some may argue that Tivozanib may be more appropriate following the availability of TIVO-3 results
- Everolimus is certainly an active treatment although it may not have the highest bar for OS
- Did everolimus ‘overperform’ in this trial? The ORR with everolimus in the LITESPARK-005 trial (3.5%) is comparable to that observed in RECORD-01 and METEOR (1 – 5%), although it is important to note that LITESPARK-005 had a slightly less favorable population compared to the other two trials

What about the experimental arm? Did belzutifan ‘underperform’ for OS? Dr. Pickering argued that this remains unclear at this point. What is clear is that belzutifan is active with a high ORR in this setting, better PFS compared to everolimus, with an impressive durability in responders. Thus, it remains unclear why the OS remains negative.
The key take home messages from LITESPARK-005 are:
- This is a large, positive study that supports the use of belzutifan for the treatment of advanced ccRCC
- It demonstrates that belzutifan is meaningfully active in pre-treated patients with advanced clear cell RCC
- PFS and OS are statistically superior to everolimus, albeit with some early progression
- The duration of response to belzutifan is impressive
- OS is not significant at this point, although we need to await the final analysis
- Belzutifan is well tolerated and QoL outcomes clearly and quickly favor belzutifan, which is important in this advanced treatment-refractory cohort of patients
What are some strategies that may further enhance belzutifan activity? Can we harness the efficacy and tolerability of belzutifan to further improve outcomes in patients with advanced clear cell RCC by escalating the dose (120 mg to 200 mg) or using the drug in combination (e.g., plus cabozantinib)?
The LITESPARK-013 study evaluated the safety and efficacy of two doses of belzutifan (120 mg and 200 mg) in patients with advanced ccRCC. Overall, no significant differences were observed in the efficacy outcomes of OS, PFS, ORR, or duration of response. While any grade and grade 3 or worse adverse events were similar for both dose groups, adverse events leading to a dose modification (58% versus 46%) or treatment discontinuation (14% versus 5%) occurred more commonly with the higher dose of 200 mg.
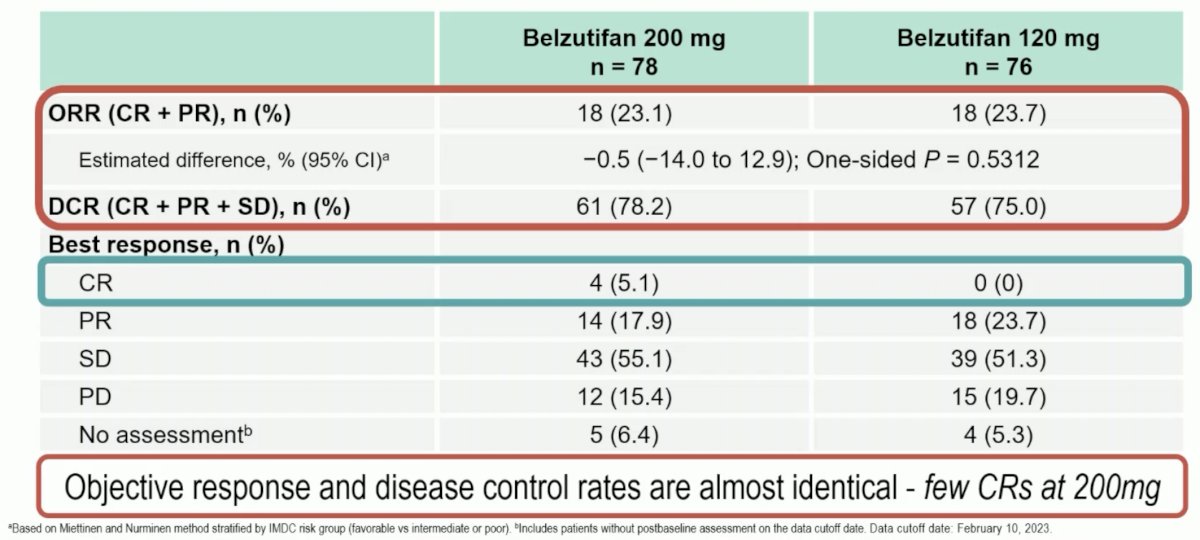
Dr. Pickering noted that while the ORR was similar in both groups (23.1 – 23.7%), 5% of patients in the 200 mg dose group had a complete response, compared to none in the 120 mg group. She again highlighted that while overall and grade 3-5 adverse events were similar, events leading to a dose modification or treatment discontinuation occurred much more frequently with the belzutifan 200 mg dose.
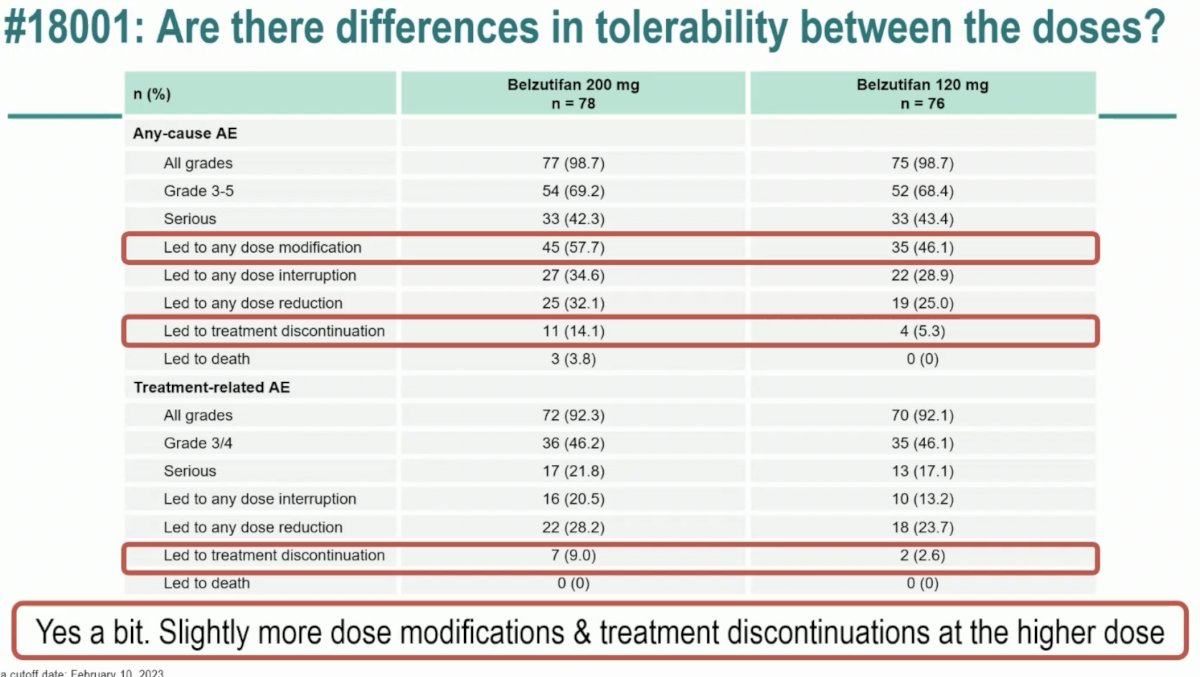
The take home messages from LITESPARK-013 were as follows:
- Dose optimization is important to ensure maximal benefit for patients
- Dose escalation of belzutifan did not improve efficacy and slightly worsened tolerability
- The currently utilized dose of 120 mg once daily continues to be recommended
- Whilst this study will not change our practice, it remains valuable as it provides confidence in the recommended dose of 120 mg being investigated in ongoing studies
What about using belzutifan in combination with other agents? This may act as a ‘proof of concept’, demonstrating that belzutifan is combinable with other agents active for advanced RCC. This may signal that there are additional, synergistic benefits with such combinations. Furthermore, the results of such combinatory trials may support the ongoing investigation of this and other future combinations.
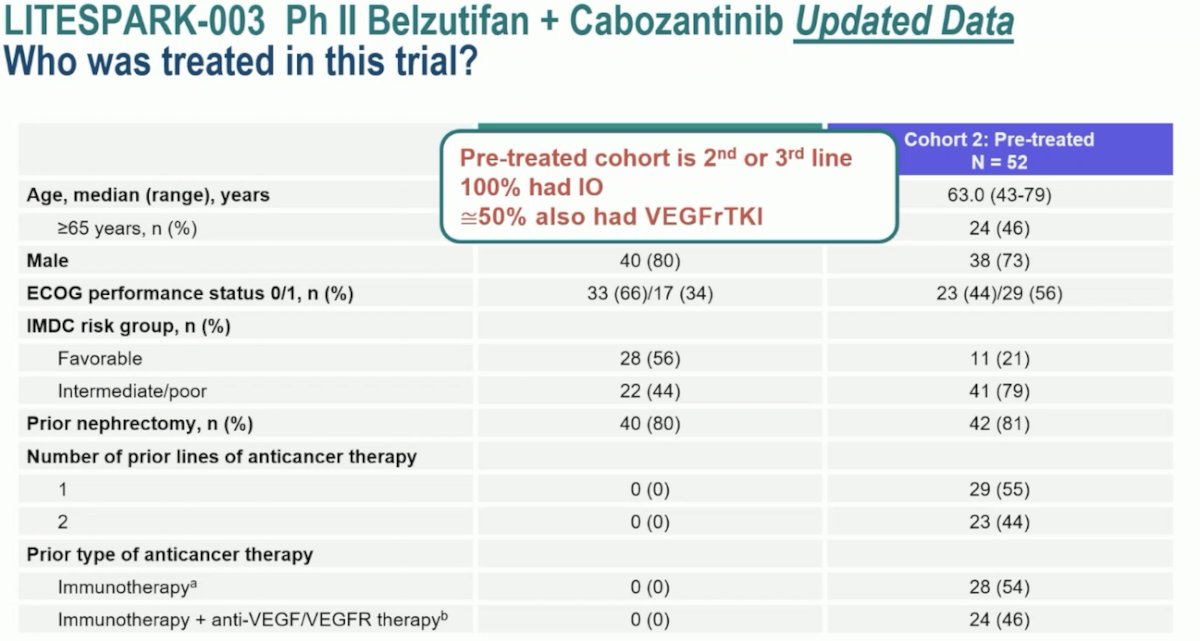
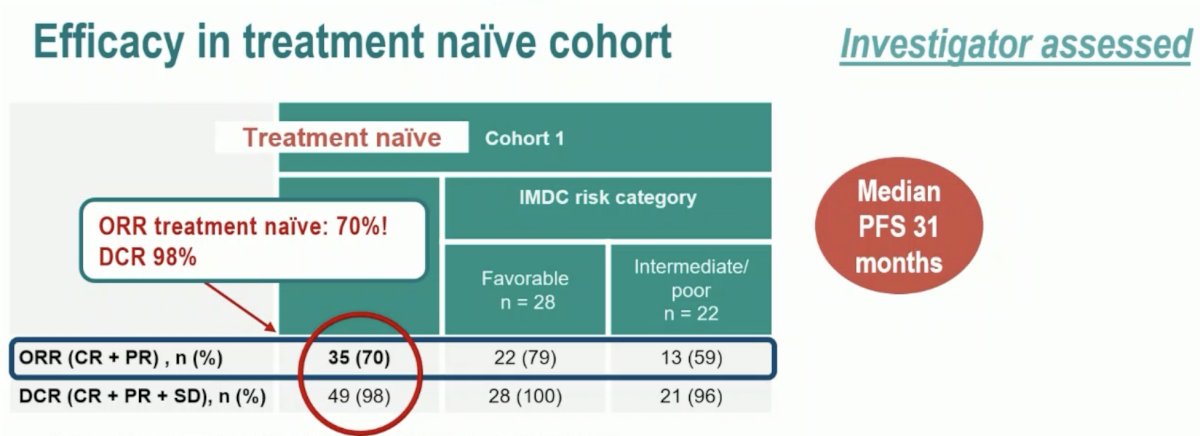
The phase 2 LITESPARK-003 trial evaluated belzutifan 120 mg once daily plus cabozantinib 60 mg orally once daily in patients who were either treatment-naïve (Cohort 1) or were previously treated (Cohort 2). This trial demonstrated an ORR of 70% in Cohort 1(4 complete and 31 partial responses), with a consistent ORR across IMDC risk categories. The median duration of response was 28.6 months, with 58% having an estimated response beyond 24 months. In Cohort 2, the ORR was 31% (2 complete and 14 partial responses) and was consistent across IMDC risk categories and number of prior lines of therapy. The median duration of response was 31.5 months, with 51% having an estimated response beyond 24 months.
Dr. Pickering noted the intriguing Cohort 1 patient population of treatment-naïve patients. These are patients that chose to forgo standard of care 1st line IO-based therapy in favor of this experimental combinatory approach of belzutifan + cabozantinib. Who are these patients? She speculated that these are likely patients with IMDC favorable risk disease (56%), with a low disease burden, who were potentially unsuitable for IO-based therapy. Conversely, Cohort 2 included heavily pre-treated patients in the 2nd or 3rd line treatment setting, of whom 100% had received IO agents and ~50% received VEGF-TKI.
As demonstrated in the waterfall plots below, most patients treated with belzutifan + cabozantinib experienced tumor shrinkage. The progressive disease rate was low, particularly in Cohort 1 (1 patient only). She noted the striking duration of response among responders, with a median duration of 28.6 months and 31.5 months in Cohorts 1 and 2, respectively.

Putting the efficacy results of the treatment-naïve Cohort 1 in perspective, Dr. Pickering noted that the ORR of 70% is impressive and similar to that observed with Lenvatinib + pembrolizumab. She noted that the median PFS of 31 months is quite long and the treatment response appears to be durable. As such, we’ll need to see whether this activity is maintained in a randomized setting.
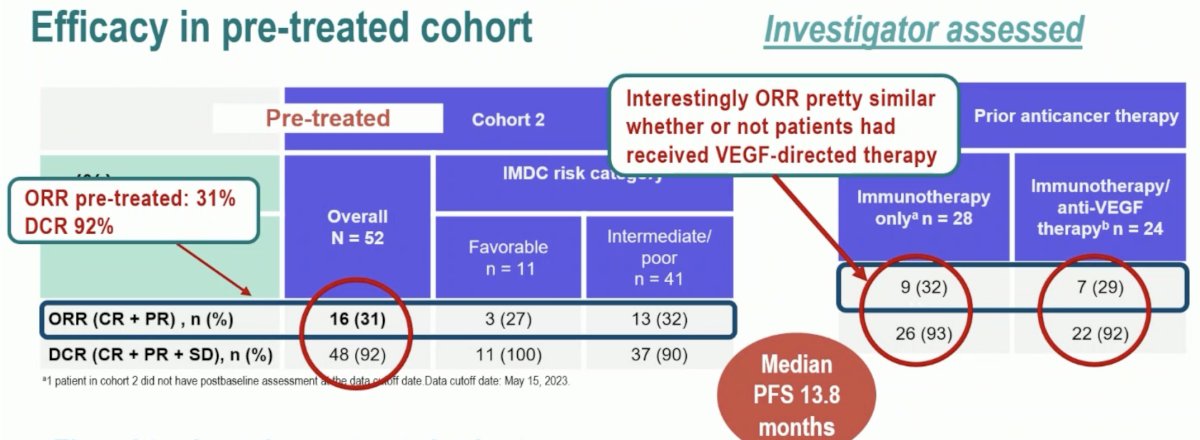
What about the pre-treated cohort? The ORR was 31% with a disease control rate of 92%. Notably, the ORR was similar irrespective of whether patients received VEGF-directed therapy or not. The response rate in this analysis appears to be consistent with that from earlier analysis of this trial. The durability in this cohort is also impressive at 31 months, and this combination appears to be active; however, how does this combination measure up compared with monotherapy?
There are two questions to be asked here:
- What is the benefit of adding cabozantinib to belzutifan in this setting?
- Conversely, what is the benefit of adding belzutifan to cabozantinib?
To address the 1st question, Dr. Pickering compared outcomes from LITESPARK-003 Cohort 2 to those from the phase 1 aRCC cohort trial of Choeiri et al. in 2021, LITESPARK-013, and LITESPARK-005. While these are different trials with different eligibility criteria, the ORR and DCR look higher, and the PFS is longer compared to the phase III LETSPARK-005 trial. So overall, it does appear that cabozantinib might be adding to the activity of belzutifan.

It is not quite clear at this point how much belzutifan is ‘adding’ to cabozantinib. Compared to the other trials summarized below, the ORR of cabozantinib + belzutifan in Cohort 2 (31%) appears to be comparable to those from METEOR, CANTATA, CONTACT-03, and CABOPOINT. It does appear, however, that the PFS and median DoR may be longer with this combination. Again, it is important to note between trial differences when comparing outcomes from these trials.
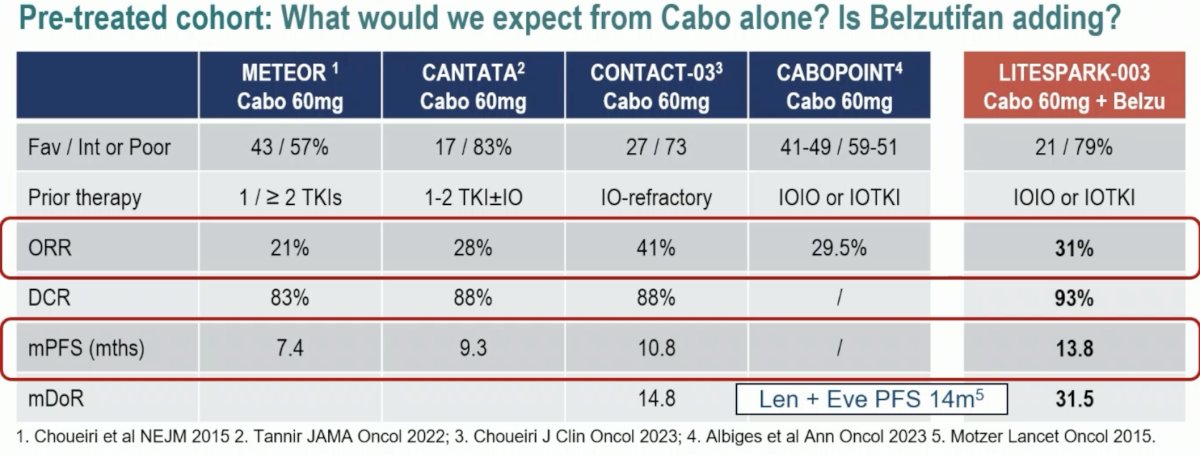
The take home messages from LITESPARK-003 are:
- Belzutifan and cabozantinib can be combined at full doses of both drugs with manageable toxicities
- This is a ‘proof of concept’ trial that demonstrates that belzutifan is combinable with other agents active in advanced RCC
- The combination was active and looked more efficacious than belzutifan alone
- It remains unclear how much activity (and toxicity) belzutifan is adding to cabozantinib monotherapy without randomized trials
- The results of this trial support the continued investigation of belzutifan combinations with other agents
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Meeting, Madrid, Spain, Fri, Oct 20 – Tues, Oct 24, 2023.
References:
- Jonasch E, Donskov F, Iliopoulos O, et al. Belzutifan for Renal Cell Carcinoma in von Hippel–Lindau Disease. N Engl J Med 2021;385:2036-2046.
- Choueiri TK, Bauer TM, Papadopoulos KP, et al. Inhibition of hypoxia-inducible factor-2α in renal cell carcinoma with belzutifan: a phase 1 trial and biomarker analysis. Nature Med 2021;27:802-805.
ESMO 2023: LITESPARK-003 Phase 2 Study of Belzutifan in Combination With Cabozantinib for Advanced Clear Cell Renal Cell Carcinoma (ccRCC)
ESMO 2023: LITESPARK-005 Belzutifan Versus Everolimus in Participants with Previously Treated Advanced Clear Cell Renal Cell Carcinoma: Randomized Open-Label Phase 3
ESMO 2023: LITESPARK-013 Phase 2- Safety and Efficacy of Two Doses of Belzutifan in Patients with Advanced Renal Cell Carcinoma (RCC)


