(UroToday.com) The 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023 was host to a non-prostate genitourinary proffered paper session. Dr. Laurence Albiges presented the late-breaking abstract results of the open-label phase III LITESPARK-005 study comparing belzutifan to everolimus in patients with previously treated advanced clear cell renal cell carcinoma (ccRCC).
The Hypoxia-Inducible Factor (HIF) pathway is central to the pathophysiology of ccRCC and von Hippel-Lindau (VHL) disease. Belzutifan, a model of bench to bedside development, is a first-in-class oral HIF-2a inhibitor that block heterodimerization with HIF-1B and downstream oncogenic pathways. Belzutifan (Welireg®) is approved in the United States for certain VHL disease associated RCC, pancreatic neuroendocrine tumors, and central nervous system hemangioblastomas. Furthermore, this drug has demonstrated clinical activity in previously treated, advanced ccRCC.1-3
LITESPARK-005 (NCT04195750) is an open label, randomized phase III study of patients with unresectable, locally advanced, or metastatic ccRCC with evidence of disease progression after 1-3 lines of prior systemic therapy, including ≥1 anti-PD-(L)1 agent and ≥1 VEGFR-TKI. Patients in this study underwent 1:1 randomization, stratified by IMDC prognostic score (0 versus 1-2 versus 3-6) and prior VEGF/VEGFR-targeted therapies (1 versus 2-3), to:
- Belzutifan 120 mg orally once daily (n=374)
- Everolimus 10 mg orall once daily (n=372)
The co-primary endpoints were progression-free survival, per RECIST v1.1, assessed via blinded independent central review (BICR), and overall survival (OS). Key secondary endpoints included objective response rate (ORR), duration of response (DOR), safety, and patient-reported outcomes.
This trial was designed with a type 1 (alpha) error rate of 0.005 for PFS, 0.019 for OS, and 0.001 for all key secondary endpoints (all 1-sided). The type 1 error rate was strongly controlled at 0.025 (1-sided). If a null hypothesis was rejected at the 1st interim analysis, no further formal testing of that hypothesis occurred. The hazard ratios and 95% confidence intervals were estimated using a stratified Cox regression model. Between-arm differences were assessed using a stratified log-rank test.
This trial randomized 746 participants between March 10, 2020, and January 19, 2022
Given the stratified nature of the randomization, the groups were well-balanced for IMDC risk categories, with ~80% of patients having IMDC intermediate to poor risk disease. 70% had undergone a prior nephrectomy. This was a heavily pre-treated patient cohort with approximately 87% of patients having received 2 – 3 prior lines of therapy.
The trial met its co-primary endpoint of BICR assessed PFS, as demonstrated in the Kaplan Meier curve below. At the landmark 18 months analysis, 22.5% of patients remained free of progression with belzutifan, compared to 9% with everolimus (HR: 0.74, 95% CI: 0.63 – 0.88). Notably, the magnitude of improvement, as quantified by the HR, remained consistent with extended follow-up at this 2nd interim analysis.
Consistent PFS results were seen when assessed by investigators:
The forest plot below shows consistent PFS benefits in all evaluated subgroups, with suggestion of a relatively improved response with belzutifan in patients who had received only one prior line of therapy.
To date, no overall survival benefits have been observed for belzutifan, compared to everolimus, in this study. While there is a signal for OS benefit (HR: 0.88, 95% CI: 0.73 - 1.07, p=0.099), with 18 months OS rates of 55.2% and 50.6% for belzutifan and everolimus, respectively, to date this has not met statistical significance.
An ORR was observed in 22.7% of belzutifan patients, compared to 3.5% for everolimus. A complete response was observed in 3.5% of belzutifan patients, compared to none with everolimus.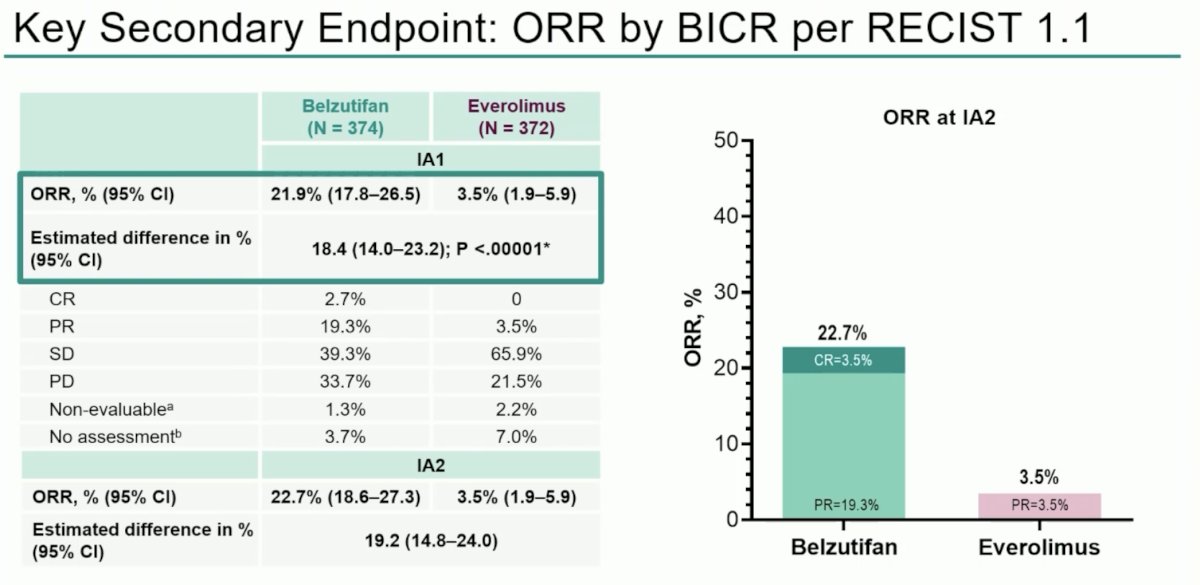
The median time to response was similar in both arms at 3.7-3.8 months; however, the median duration of response was longer with belzutifan (19.5 versus 13.7 months).
With regards to safety outcomes, grade 3 or worse events were similar in both arms (~62%). However, Dr. Albiges highlighted that adverse events leading to treatment discontinuation occurred in 6% of belzutifan patients, compared to 15% of those receiving everolimus.
The most common adverse events were anemia and fatigue, with approximately 30% of patients having grade 3 or worse anemia in the belzutifan arm.
With regards to patient-reported outcomes, evaluated using the FKSI-DRS and QLQ-C30 GHS/QoL questionnaires, patients receiving belzutifan had significantly improved time to confirmed deterioration.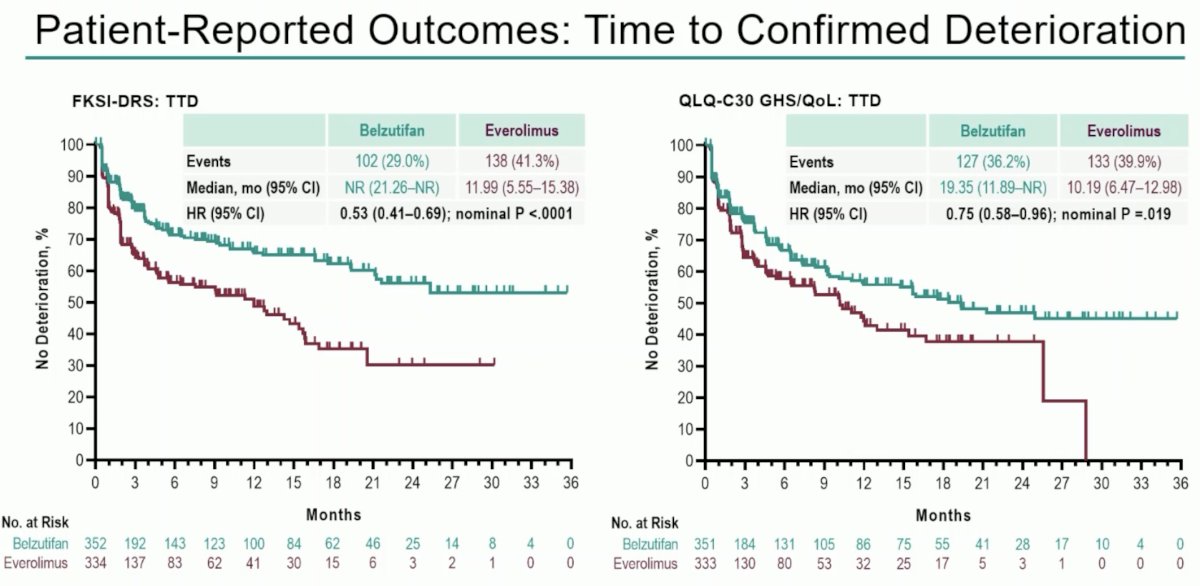
Dr. Albiges concluded her presentation as follows:
- LITESPARK-005 establishes HIF-2a inhibition as a novel therapeutic mechanism of action in advanced clear cell RCC
- Belzutifan demonstrated a statistically significant improvement in progression-free survival and objective response rate versus everolimus
- There appears to be a 25% reduction in risk for progression or death with belzutifan versus everolimus
- Overall survival difference has not reached statistical significance; final analysis is pending
- Belzutifan was well tolerated, and adverse events were consistent with the known safety profile of the drug
- Quality of life as assessed by FKSI-DRS and QLQ30 GHS/QoL favored belzutifan
- Belzutifan is currently being studied in combination in second-line, first-line, and adjuvant settings in phase 3 studies
- LITESPARK-005 is the first positive phase 3 study in patients with advanced kidney cancer following immune checkpoint and anti-angiogenic therapies
Presented by: Laurence Albiges MD, PhD, Professor, Medical Oncology, Vice Chair of the Department of Cancer Medicine at the Gustave Roussy Institute, Villejuif, France
Written by: Rashid K. Sayyid, MD, MSc – Society of Urologic Oncology (SUO) Clinical Fellow at The University of Toronto, @rksayyid on Twitter during the 2023 European Society of Medical Oncology (ESMO) Annual Congress held in Madrid, Spain between October 20th and 24th, 2023
References:- Jonasch et al. Belzutifan for Renal Cell Carcinoma in von Hippel-Lindau Disease. N Engl J Med 2021;385:2036-2046.
- Chouieri et al. Inhibition of Hypoxia-Inducible Factor-2α in Renal Cell Carcinoma with Belzutifan: A Phase 1 Trial and Biomarker Analysis. Nat Med 2021;27:802-805.
- Chouieri et al. Lenvatinib plus pembrolizumab versus sunitinib as first-line treatment of patients with advanced renal cell carcinoma (CLEAR): extended follow-up from the phase 3, randomised, open-label study. Lancet Oncol 2023;24:553-562.Chouieri et al. Lenvatinib plus pembrolizumab versus sunitinib as first-line treatment of patients with advanced renal cell carcinoma (CLEAR): extended follow-up from the phase 3, randomised, open-label study. Lancet Oncol 2023;24:553-562.


