Notably, as a part of the multi-arm, multi-stage STAMPEDE platform, the primary analysis assessing abiraterone acetate included a range of patients with non-metastatic and metastatic hormone-sensitive prostate cancer. In contrast, the LATITUDE trial enrolled only men with high-risk metastatic disease (3+ bone metastases, visceral metastases, or Gleason score 8 or greater).
In a proffered paper presentation at this year’s European Society of Medical Oncology (ESMO) 2020 Virtual Annual Meeting, Dr. Nicholas James presented updated, long-term results of the STAMPEDE Arm G comparison examining abiraterone acetate plus prednisolone in addition to ADT, as compared to ADT alone, among men with metastatic hormone-sensitive prostate cancer.
To briefly summarize, the STAMPEDE Arm G to Arm A comparison is based on a 1:1 randomization between the standard of care (androgen deprivation therapy) and standard of care plus abiraterone acetate (1000mg PO daily) and prednisolone 5mg PO daily. Treatment was continued until PSA, radiological, or clinical progression. The primary outcome was overall mortality and, in this analysis, data was frozen at 3 years following primary survival results. Analyses were performed using Cox proportional hazards models and flexible parametric models after adjusting for baseline stratification factors.
In this analysis with a database lock of April 3, 2020, 1003 patients with metastatic hormone-sensitive prostate cancer among a total of 1917 who were contemporaneously randomized to abiraterone acetate in addition to standard of care or standard of care alone. Accrual occurred between November 2011 and January 2014. Among the included patients, the median age was 67 years, 48% had a high metastatic burden of disease, 94% had newly diagnosed disease and median PSA was 97 ng/mL.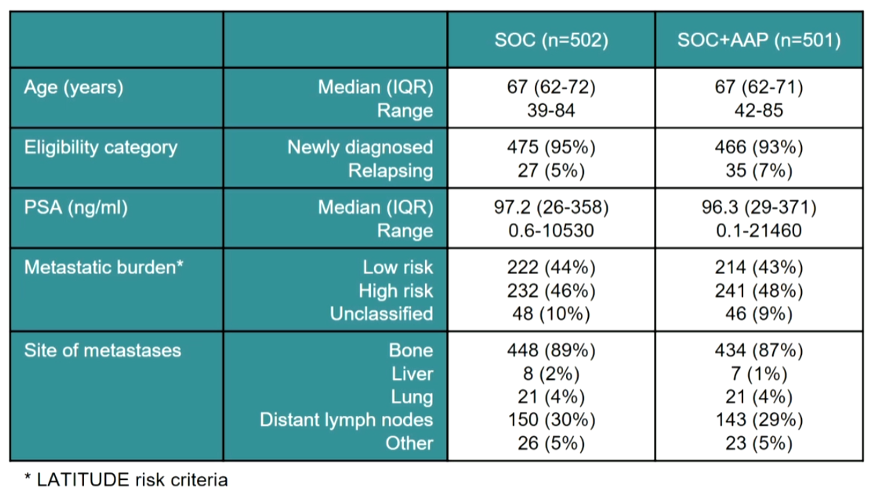
In this updated analysis, the median follow-up was 6.1 years (up from 3.3 years at the time of initial analysis. At this point, a total of 329 deaths were observed among patients randomized to standard of care (ADT alone) and 244 among those randomized to abiraterone acetate in addition to ADT. Five-year overall survival was 60% among those randomized to abiraterone acetate and ADT as compared to 41% among those receiving ADT alone. This corresponded to a 40% relative improvement in survival (hazard ratio 0.60, 95% confidence interval 0.50 to 0.71). In absolute terms, median survival was 6.6 years among those receiving abiraterone acetate and ADT as compared to 3.8 years among men receiving ADT alone, an absolute median survival benefit of 2.8 years.
Dr. James then presented data for failure-free survival which similarly demonstrated a significant improvement for patients receiving abiraterone acetate (hazard ratio 0.34, 95% confidence interval 0.29 to 0.40) with more than 3 years absolute additional failure-free survival benefit for those randomized to abiraterone acetate (4.3 versus 0.96 years). Progression-free survival was similarly improved (hazard ratio 0.58, 95% confidence interval 0.49 to 0.69) with an absolute survival difference of 2.5 years. The risk of skeletal-related events was also lower among those receiving abiraterone acetate (hazard ratio 0.56, 95% confidence interval 0.41 to 0.76).
The relative benefit in overall survival of abiraterone acetate was similar among patients with a low-risk disease according to the LATITUDE criteria (hazard ratio 0.55, 95% confidence interval 0.41 to 0.76) and those with high-risk disease (hazard ratio 0.54, 95% confidence interval 0.43 to 0.69).
Similarly, an overall survival benefit was seen when stratifying according to CHAARTED volume criteria in those with low volume disease (hazard ratio 0.53, 95% confidence interval 0.38 to 0.74) and those with high volume disease (hazard ratio 0.59, 95% confidence interval 0.47 to 0.74).
Toxicity in this updated analysis was similar to previous reports with 16% of patients experiencing severe (grade 3 or greater) toxicity.
The authors conclude that, in patients with metastatic hormone-sensitive prostate cancer, the addition of abiraterone acetate to ADT demonstrates sustained and significant improves in overall survival, with no significant differences on the basis of disease volume. Notably, this analysis shows that the benefit of abiraterone acetate does not attenuate over time, unlike the benefit of docetaxel in this disease space.
Presented by: Professor Nicholas James, MBBS, FRCP, FRCR, Ph.D., Professor of Clinical Oncology at the Institute of Cancer Research at Royal Marsden Hospital, London
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center, Contact: @WallisCJD on Twitter at the 2020 European Society for Medical Oncology Virtual Congress (#ESMO20), September 19th-September 21st, 2020.
Related Content:
Quality of Life in the Treatment of mHSPC: The STAMPEDE Trial - Hannah Rush
ASCO GU 2020: Comparative Quality of Life in Patients Randomized Contemporaneously to Docetaxel or Abiraterone In the STAMPEDE Trial
STAMPEDE Trial - Comparative Quality of Life in Patients Randomized Contemporaneously to Docetaxel of Abiraterone
Abiraterone Acetate plus Prednisolone for Hormone-Naïve Prostate Cancer: Long-Term Results from Metastatic (M1) Patients in the STAMPEDE Randomized Trial - Nicholas James


