- LBA24 - TROPHY-U-01 Cohort 1 Final Results: A Phase 2 Study of Sacituzumab Govitecan (SG) in Metastatic Urothelial Cancer (mUC) That Has Progressed After Platinum (PLT) and Checkpoint Inhibitors (CPI) – Presented by Dr. Yohann Loriot (Villejuif, France)
- 698O - Patient-reported outcomes (PROs) from IMvigor130: a global, randomised, partially blinded Phase III study of atezolizumab (atezo) + platinum-based chemotherapy (PBC) vs. placebo (PBO) + PBC in previously untreated locally advanced or metastatic urothelial carcinoma (mUC) – presented by Dr. Aristotelis Bamias (Athens, Attiki, Greece)
- 699O - Avelumab first-line (1L) maintenance + best supportive care (BSC) vs. BSC alone for advanced urothelial carcinoma (UC): association between clinical outcomes and exploratory biomarkers. Presented by Dr. Srikala Sridhar (Toronto, Canada).
Dr. Gschwend began his talk stating that mUC is not a curable disease in most cases, and patients rarely survive beyond five years. Treatment of this disease is associated with a high therapy burden and has significant treatment-related adverse events (TRAEs) with increasing costs.
The evolution of the treatment options is shown in Figure 1, showing many new treatment options that are becoming available.
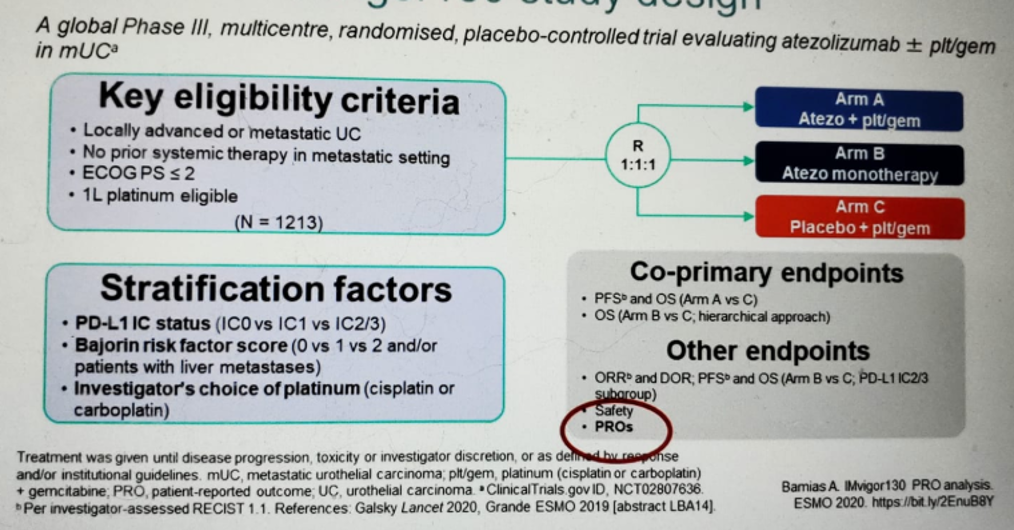
IMvigor130 was the first abstract discussed (figure 2). This was a positive randomized controlled trial, meeting the primary endpoint of progression-free survival (PFS), showing a . The analysis of overall survival (OS) is still pending. Atezolizumab + platinum-based chemotherapy was well-tolerated with no relevant toxicity added resulting from the addition of atezolizumab to chemotherapy.
IMvigor130 also provided an analysis of quality of life and patient-reported outcomes (PROs), which, according to Dr. Gschwend, provide an overall benefit. There was no significant difference in function and quality of life domains in both treatment arms. The PROs were not compromised by the addition of atezolizumab to the standard platinum-based chemotherapy.
Figure 1 – Evolution of systemic treatment options in metastatic urothelial carcinoma: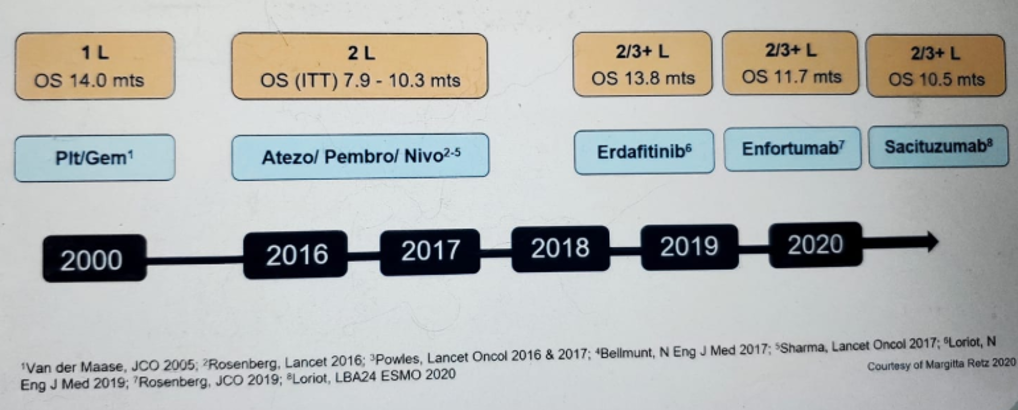
Figure 2 – Imvigor 130 trial NCT02807636 design:
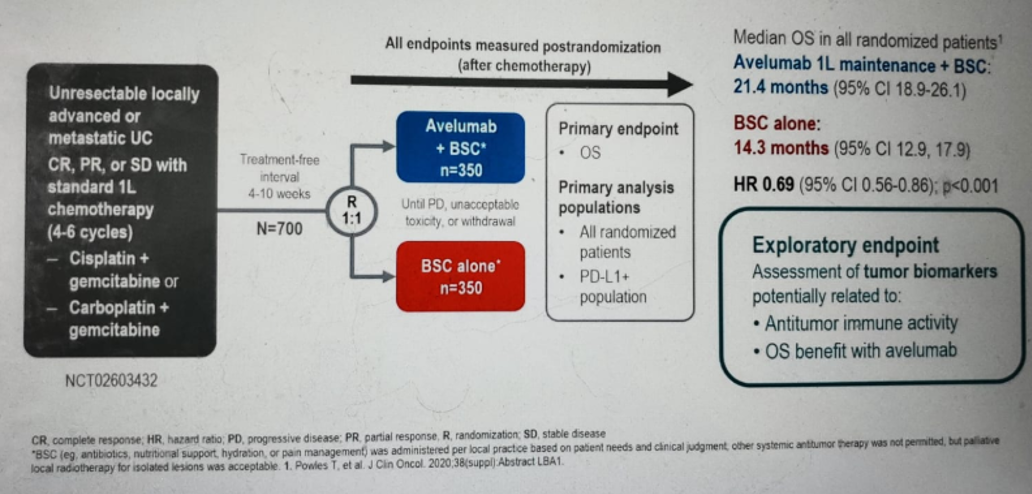
Next, Dr. Gschwend discussed the JAVELIN bladder 100 phase three trial comparing the best standard of care (BSC) to Avelumab maintenance + BSC in mUC patients previously treated with platinum-based chemotherapy (figure 3).
Figure 3 – JAVELIN bladder 100 trial design:
Dr. Gschwend continued and congratulated the authors of this study due to the very impressive biomarker analyses that were conducted in this study. However, it is still not clear if the work done with the suggested biomarkers will influence clinical decision making. The study shows that the impact of PD-L1 in the JAVELIN bladder 100 trial is questionable because both treatment arms did better in patients with positive PD-L1.
When assessing the impact of the tumor mutational burden (TMB) as a potential biomarker, it showed no clear prediction in this trial. In a similar manner, there was no clear prediction by either TMB or PD-L1 in the IMvigor 130 study. The other biomarkers that were identified and analyzed in the trial, including gene expression signatures, were shown to be potentially predictive, but they were more hypothesis-generating than clinically relevant. None of these potential biomarkers were shown to be predictive in the outcome of overall survival (OS) in the JAVELIN bladder 100 trial and IMvigor 130 trial.
An important limitation to remember is that the backbone of a biomarker test is the tumor tissue itself. However, it is not clear which tissue should be utilized – whether tissue should be collected from the primary tumor or from a metastasis. The timing of tissue sampling is also not clear, and tumor heterogeneity within the same tumor also raises concern.
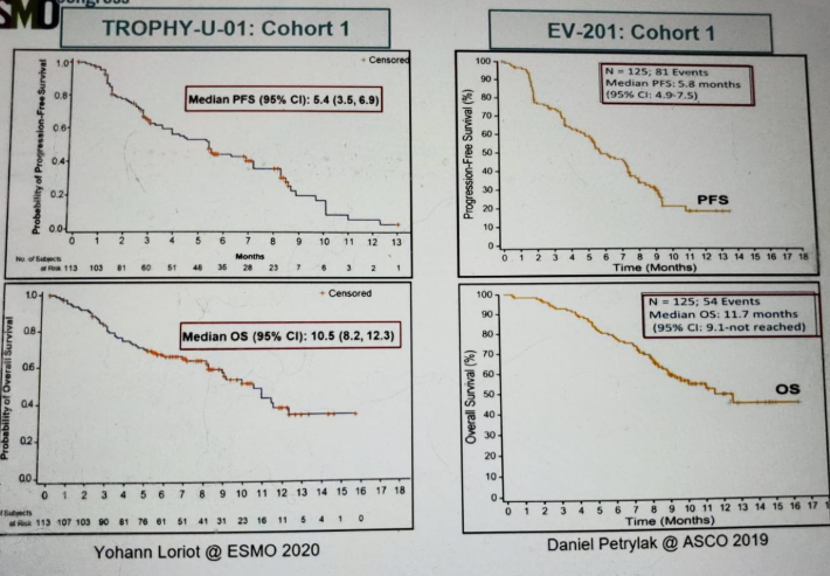
The last abstract discussed was the TROPHY-U-01 trial (figure 4), assessing the role of Sacituzumab Govitecan (SC), which is an anti-Trop-2 antibody-drug conjugate. This trial was compared to the EV-201 trial assessing the role of enfortumab Vedotin (EV), another antibody-drug conjugate, in the same patient population1. Both trials had similar results, as shown in figure 5.
The TROPHY-U-01 showed that SC has promising activity with an objective response rate of 27% compared to 44% for EV. There was a high median OS with 10.5 months for SG and 11.7 months for EV. There are currently ongoing phase three trials assessing the role of SG.
Figure 4 – TROPHY-U-01 trial NCT03547973 design:
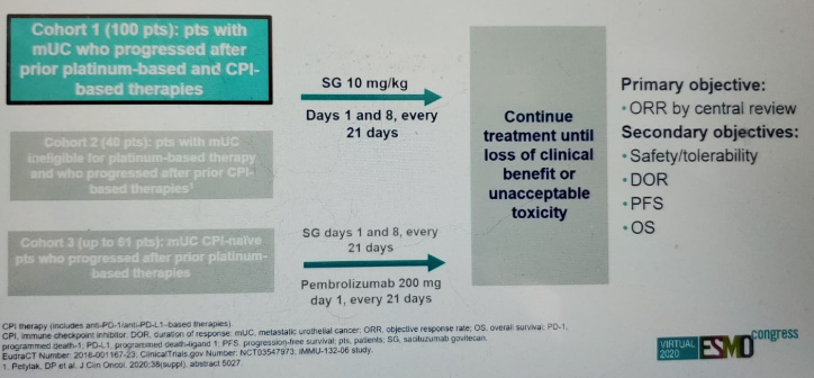
Figure 5 – Comparison of results from the TROPHY-U-01 trial and the EV-201 trial:
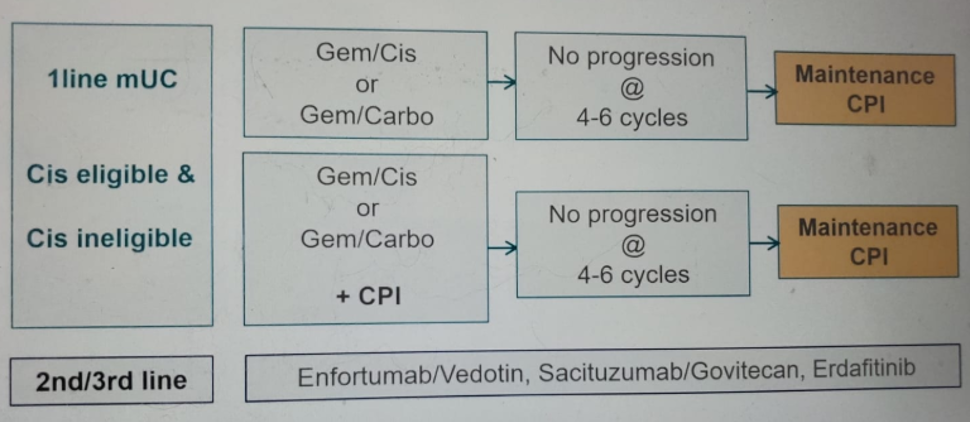
Dr. Gschwend ended his talk showing the currently available options for first-line treatment of mUC patients in 2020 and beyond (Figure 6).
Figure 6 – First-line treatment options for metastatic urothelial carcinoma in 2020 and beyond:
Presented by: Prof. Dr. Jürgen Gschwend, PhD, Department of Urology, TUM School of Medicine, Munich, Germany
Written by: Hanan Goldberg, MD, MSc., Assistant Professor of Urology, SUNY Upstate Medical University, Syracuse, NY, USA, @GoldbergHanan at the European Society for Medical Oncology Virtual Congress, ESMO Virtual Congress 2020 #ESMO20, 18 Sept - 21 Sept 2020.
References:
1. Rosenberg JE, O'Donnell PH, Balar AV, et al. Pivotal Trial of Enfortumab Vedotin in Urothelial Carcinoma After Platinum and Anti-Programmed Death 1/Programmed Death Ligand 1 Therapy. Journal of clinical oncology : official journal of the American Society of Clinical Oncology 2019; 37(29): 2592-600.
Related Content:
ASCO 2020: JAVELIN Bladder 100 Phase III Results: Maintenance Avelumab + Best Supportive Case vs BSC Alone After Platinum-Based First-Line Chemotherapy in Advanced Urothelial Carcinoma
JAVELIN Bladder 100: Avelumab for Previously Untreated Locally Advanced or Metastatic Urothelial Carcinoma - Thomas Powles
The Dynamic Advancements in Personalized Treatments for Metastatic Urothelial Cancer - Andrea Apolo


