(UroToday.com) Dr. Yohann Loriot presented the final results of the TROPHY-U-01 study, which was a phase 2 study of Sacituzumab Govitecan (anti-Trop-2) in metastatic urothelial carcinoma (mUC) patients who have progressed after platinum and checkpoint inhibitor therapy.1
Trop-2 is an epithelial cell surface antigen highly expressed in urothelial carcinoma.
Sacituzumab Govitecan (SG) (Figure 1) is distinct from other antibody-drug conjugates (ADC) since it has a high drug to antibody ratio and a bystander effect due to hydrolyzable linker hydrolysis. It has been shown to have significant activity in many tumors2,3. It has also shown a high 31% objective response rate (ORR), with a median progression-free survival (PFS) of 7.3 months, with median overall survival (OS) of 16.3 months with a manageable toxicity profile in the mUC cohort of the IMMU-132-01 trial (NCT01631552). There currently is a phase 3 trial in mUC that is underway.
Figure 1 - Sacituzumab Govitecan: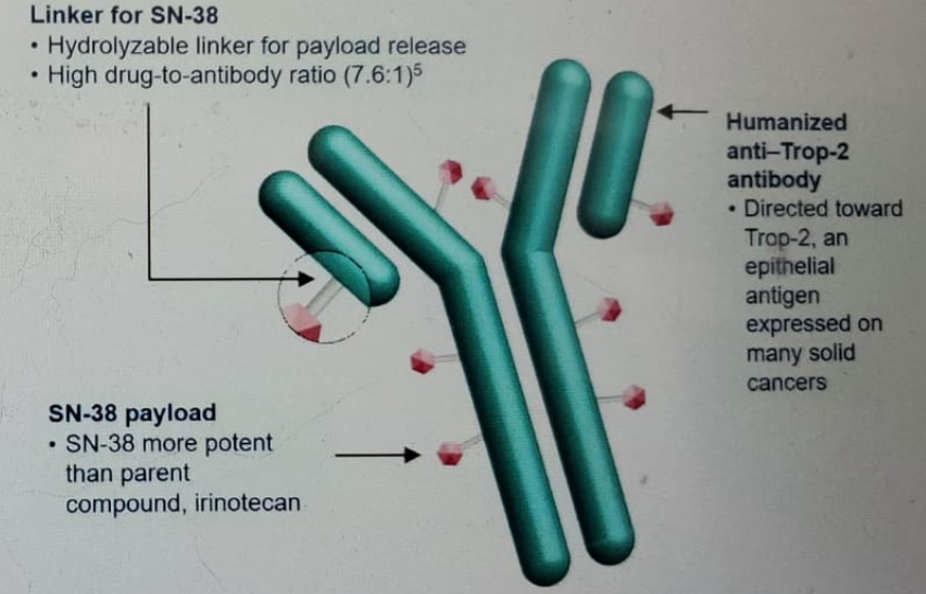
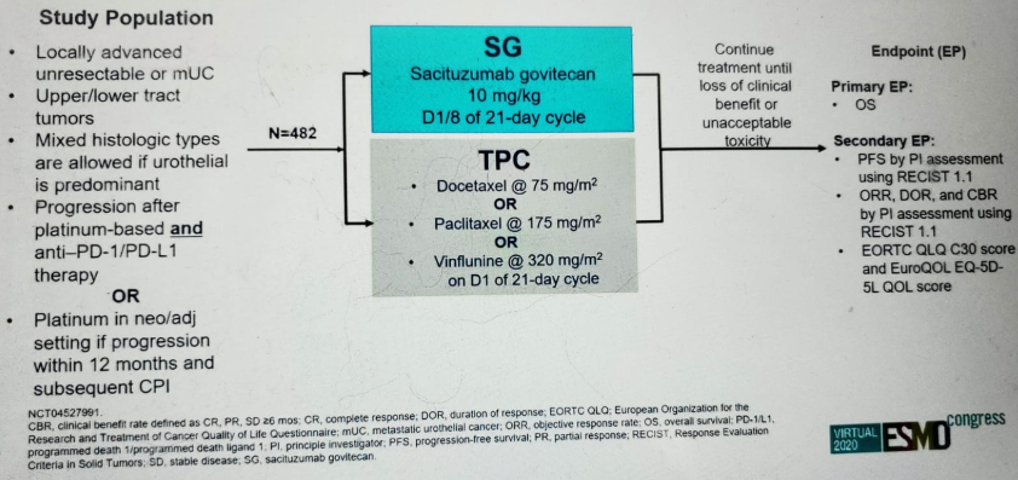
The TROPHY-U-01 study design is shown in figure 2. This study included patients with mUC who progressed after prior platinum-based chemotherapy and immunotherapy-based treatments, who were given SG 10 mg/kg on days 1 and 8, every 21 days. The patient disposition is shown in figure 3, and the patient demographics and baseline characteristics are shown in figure 4.
Figure 2 – TROPHY study design: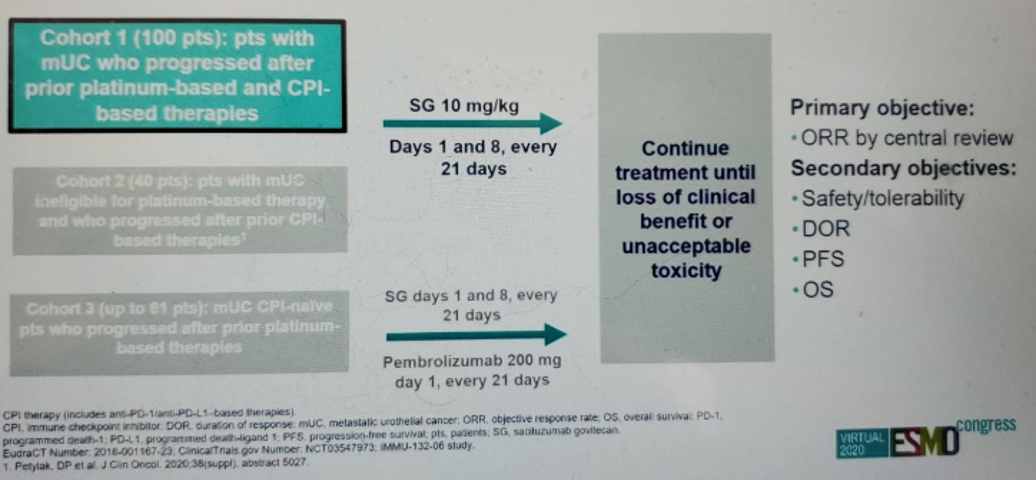
Figure 3 – Study patient disposition:
Figure 4 – Demographics and baseline characteristics: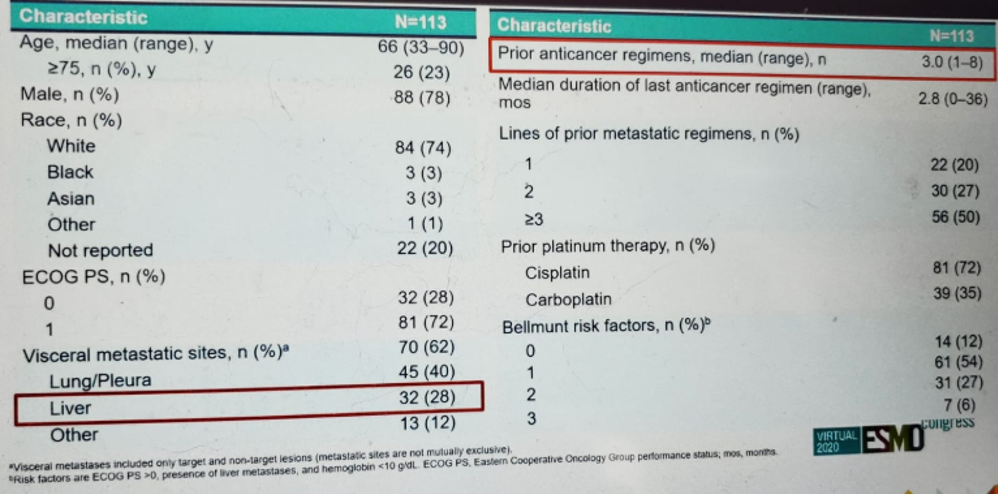
The results presented in this presentation showed an ORR of 27% with a complete response in 5% and partial response in 22% of patients. The median duration of response was 5.9 months, with a median time to onset of the response being 1.6 months. The reduction in tumor size and individual patient responses are shown in figures 5 and 6, respectively. The Kaplan-Meier survival graph is shown in figure 7, demonstrating a median PFS of 5.4 months (95% CI 3.5-6.9), and figure 8, showing a median OS of 10.5 months (95% CI 8.2-12.3).
Figure 5 – Reduction in tumor size: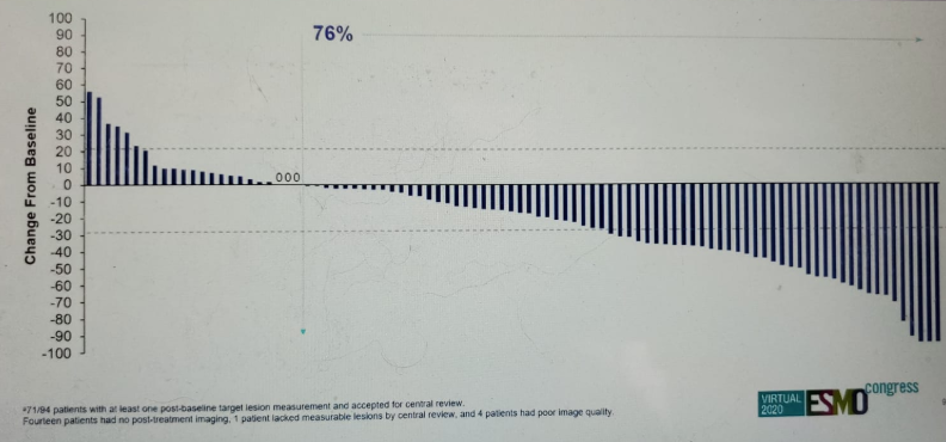
Figure 6 – Patient responses: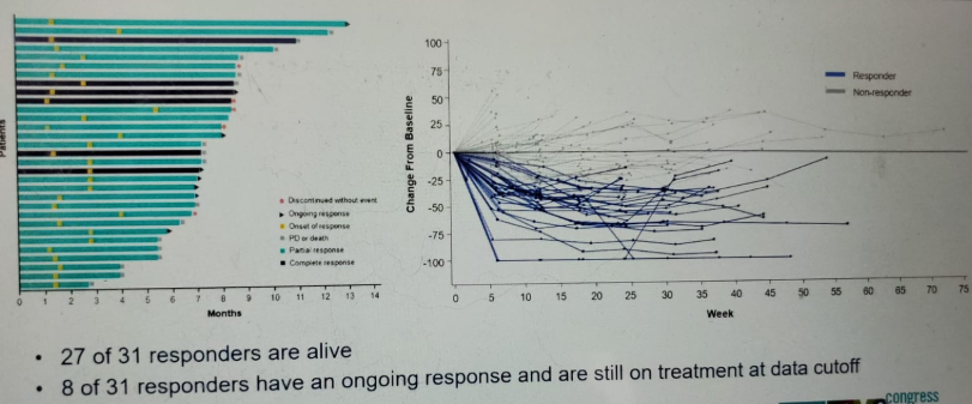
Figure 7 – PFS outcome: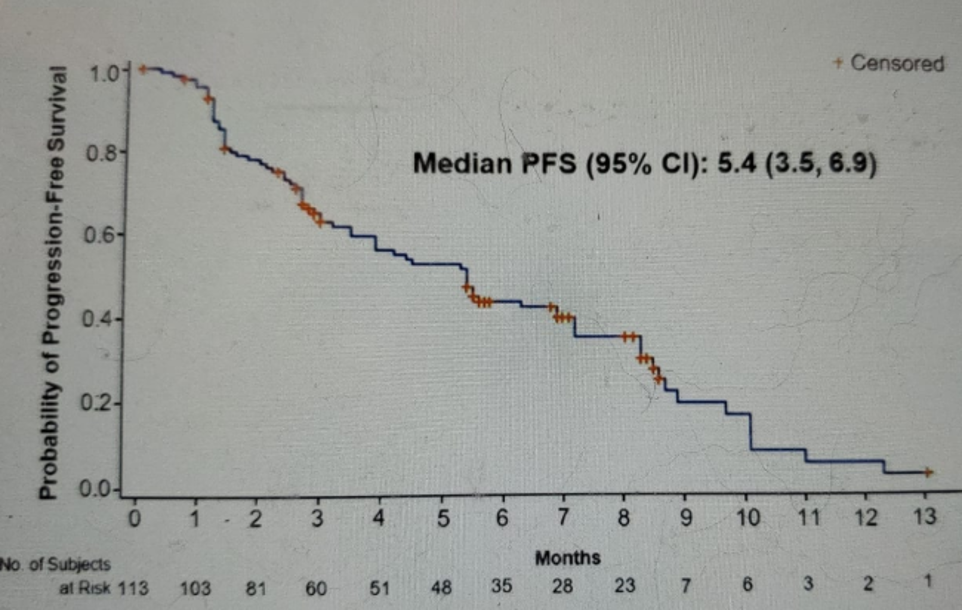
Figure 8 – OS outcome: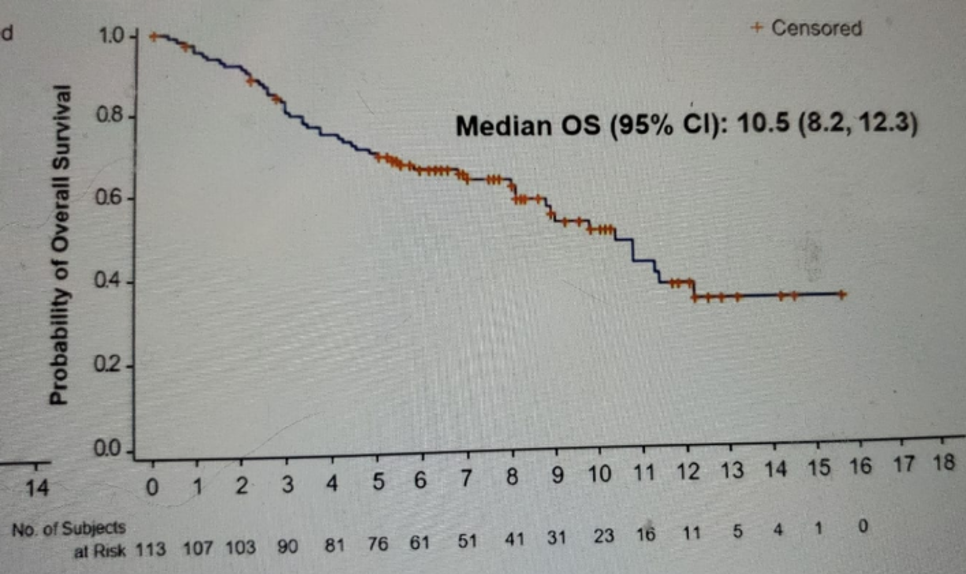
The treatment-related adverse events (TRAEs) of more than 20% of any grade and more than 5% of grade 3 or above are shown in figure 9. A total of 7 patients discontinued the study due to TRAEs, and 30% of patients required the use of GCSF. There was one treatment-related death (sepsis due to febrile neutropenia).
Figure 9 - Treatment-related adverse events: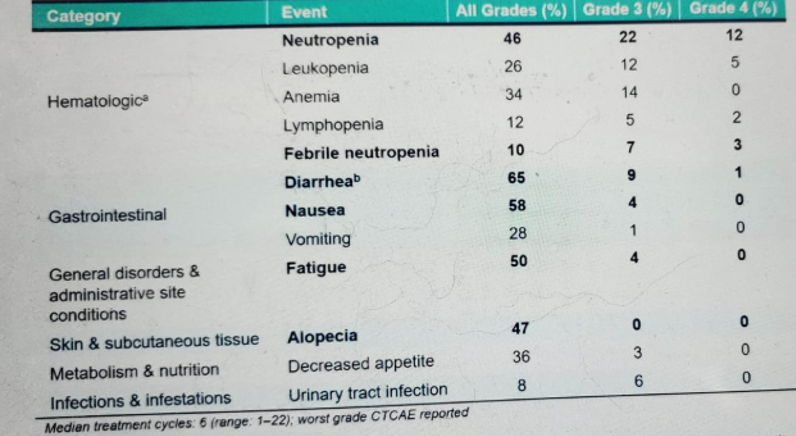
Dr. Loriot concluded his presentation reiterating that TROPHY-U-01 study confirmed the interim findings and prior phase 1 and 2 studies. The results showed that SG has significant activity and is safe in patients with heavily pretreated mUC, who progressed on both platinum-based chemotherapy and immunotherapy. The results of SG compare favorably with single-agent chemotherapy. These favorable results have led to SG having received fast track designation in this mUC indication and may have the potential to change practice in this setting. Dr. Loriot ended his talk mentioning the currently ongoing phase 3 confirmatory trial in mUC, the TROPICS-04 (NCT04527991) (Figure 10).
Figure 10 – The phase 3 TROPICS-04 trial:
References:
1. Avellini C, Licini C, Lazzarini R, et al. The trophoblast cell surface antigen 2 and miR-125b axis in urothelial bladder cancer. Oncotarget 2017; 8(35).
2. Cardillo TM, Govindan SV, Sharkey RM, Trisal P, Goldenberg DM. Humanized anti-Trop-2 IgG-SN-38 conjugate for effective treatment of diverse epithelial cancers: preclinical studies in human cancer xenograft models and monkeys. Clinical cancer research: an official journal of the American Association for Cancer Research 2011; 17(10): 3157-69.
3. Bardia A, Mayer IA, Vahdat LT, et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. New England Journal of Medicine 2019; 380(8): 741-51.
Presented by: Yohann Loriot, MD, PhD, of the Institut Gustave Roussy and University of Paris-Saclay, Villejuif, France
Written by: Hanan Goldberg, MD, MSc., Assistant Professor of Urology, SUNY Upstate Medical University, Syracuse, NY, USA, @GoldbergHanan at the European Society for Medical Oncology Virtual Congress, ESMO Virtual Congress 2020 #ESMO20, 18 Sept - 21 Sept 2020


