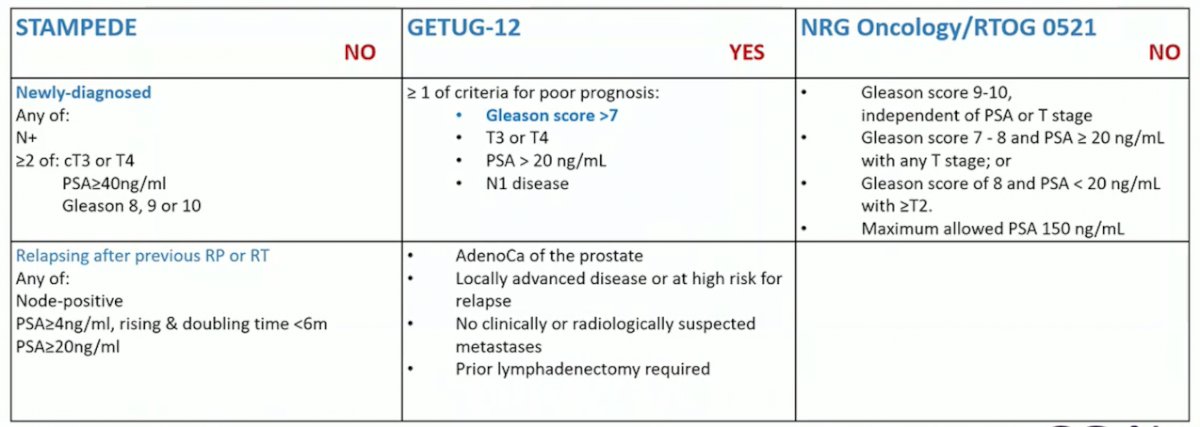
(UroToday.com) The 2023 EAU annual meeting included a joint session of the EAU and the Advanced Prostate Cancer Consensus, featuring a presentation by Dr. Maria De Santis discussing what systemic therapy and for how long we should be treating patients with high-risk localized disease, M0 on conventional imaging but one bone lesion on PSMA PET/CT. Dr. De Santis started by commenting that there are several options for how to consider a patient with high-risk localized Disease, M0 on conventional imaging, but one bone lesion on PSMA PET/CT. The first option is to consider the patient localized high risk disease (cT1c, Gleason 8, PSA 7 ng/mL), which would place the patient in the inclusion criteria for the GETU-12 trial:
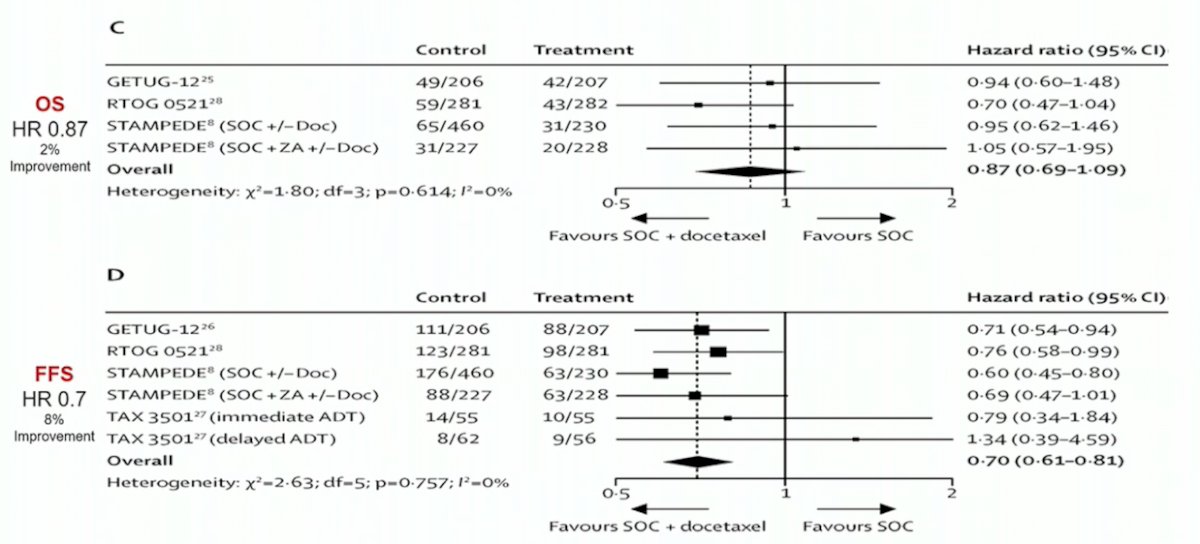
Looking at data from a 2016 systematic review assessing the addition of docetaxel or bisphosphonates to standard of care in men with localized or mHSPC,1 there is a benefit to the addition of docetaxel for failure free survival, but no survival benefit in these patients:
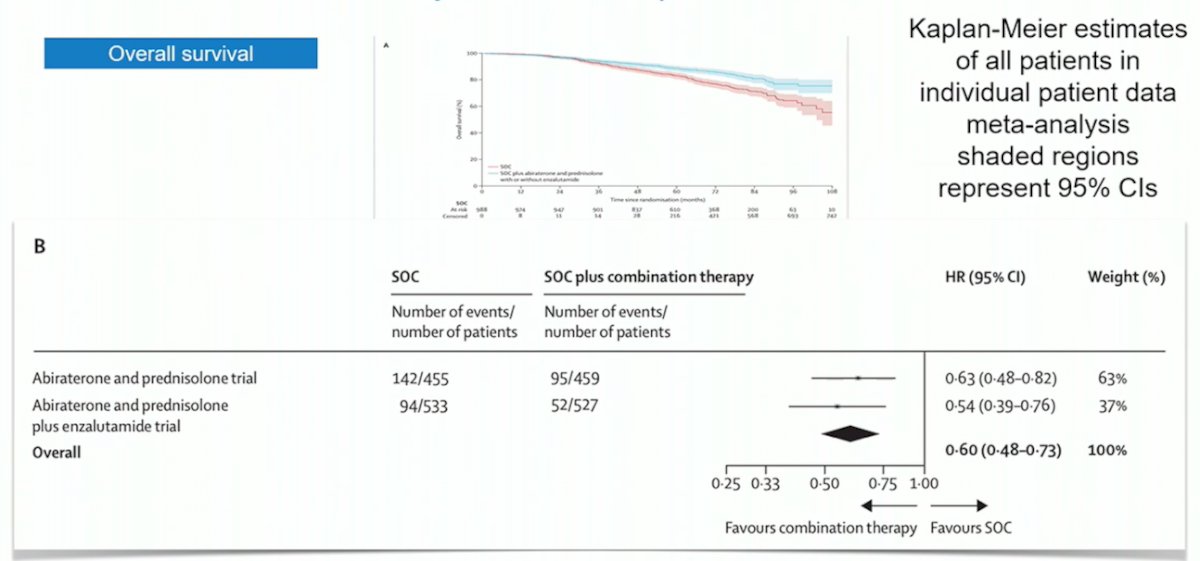
In the STAMPEDE arm of high-risk localized prostate cancer treated with either abiraterone or enzalutamide, there was a consistent effect with ARPI regardless of metastatic burden.2 Local radiotherapy (74 Gy in 37 fractions to the prostate and seminal vesicles or the equivalent using hypofractionated schedules) was mandated for node negative and encouraged for node positive disease. There were 1,974 patients randomized and over a median follow-up of 72 months (IQR 60–84), metastasis-free survival was significantly longer in the combination-therapy groups (median not reached, IQR NE–NE) than in the control groups (not reached, 97–NE; HR 0.53, 95% CI 0.44–0.64). Overall survival (median not reached [IQR NE–NE] in the combination-therapy groups vs not reached [103–NE] in the control groups; HR 0.60, 95% CI 0.48–0.73):
The EAU guidelines reflect the above findings, with a strong recommendation for prescribing 2 years of abiraterone when offering IMRT/VMAT + IGRT to the prostate plus pelvis (for cN1) in combination with long-term ADT, for M0 patients with cN1 or >2 high-risk factors (cT3-T4, Gleason >=8, or PSA >= 40 ng/mL).
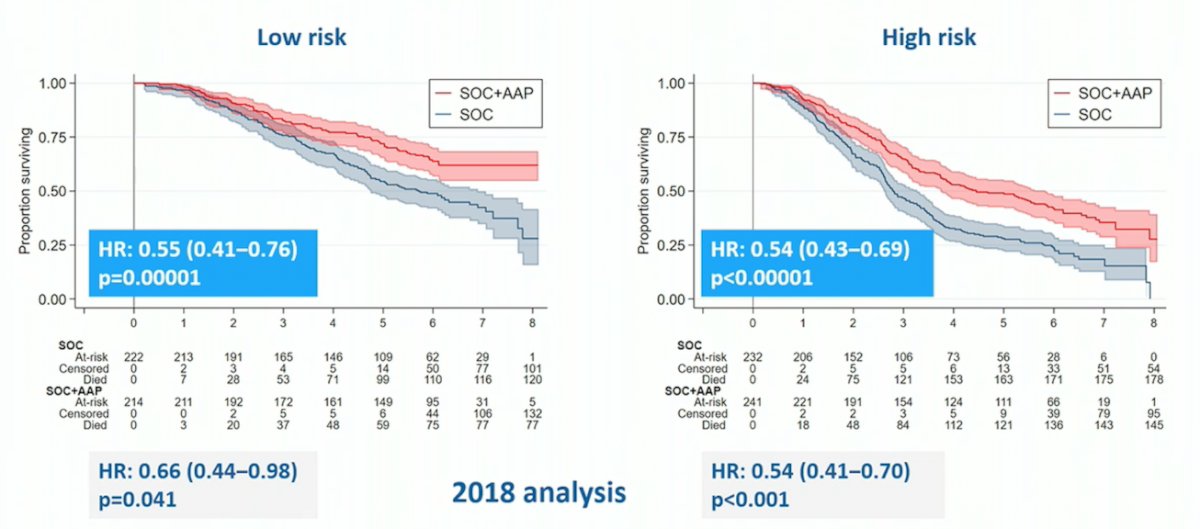
The second option for considering this patient, is them having synchronous, low volume metastatic disease (according to PSMA PET/CT). This opens up additional treatment options beyond ADT, including ADT + ARPI (abiraterone/apalutamide/enzalutamide), ADT + docetaxel, and ADT + docetaxel + abiraterone/darolutamide. Overall survival data from STAMPEDE arm G (abiraterone + prednisone + ADT vs ADT) stratified by disease burden demonstrated a survival benefit for the abiraterone arm for both low and high risk patients:
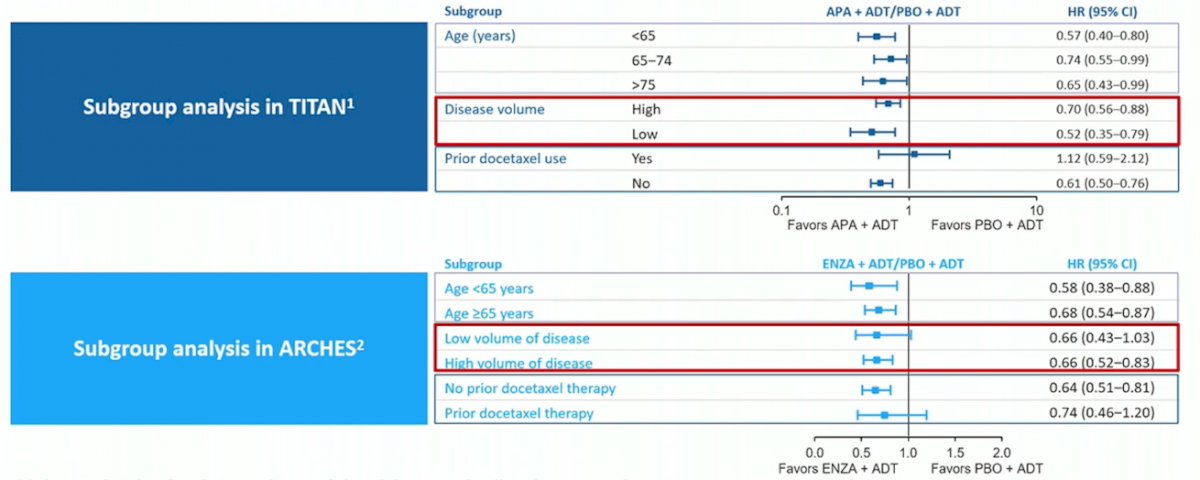
Additionally, subgroup analysis of overall survival in the TITAN (apalutamide) and ARCHES (enzalutamide) show low-volume benefit for apalutamide but not for enzalutamide:
Dr. De Santis then highlighted one of the key questions asked to the APCCC 2022 experts: If you voted for systemic therapy plus local treatment for the majority of patients with low volume/oligometastatic synchronous mHSPC (ie. 1-3 bone lesions on next generation imaging), what is your treatment recommendation? 89% of respondents said they would use ADT + an AR pathway inhibitor.
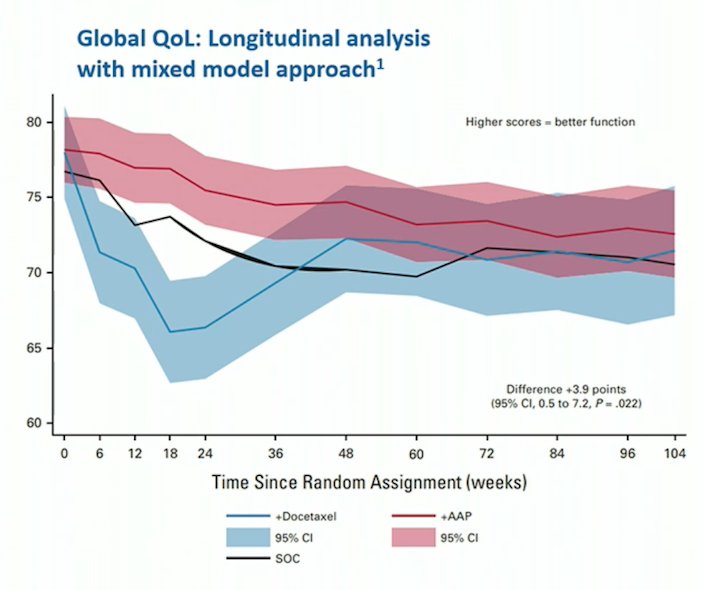
Among 515 patients concurrently randomized to standard of care + abiraterone/prednisolone or standard of care + docetaxel in STAMPEDE, Rush and colleagues found that the mean modeled global quality of life scores are +3.9 points (95% CI 0.5 to 7.2, p = 0.022) higher over 2 years in patients treated with abiraterone compared to those treated with docetaxel.3 Furthermore, patients receiving abiraterone reported higher clinically meaningful global quality of life scores in the first year:
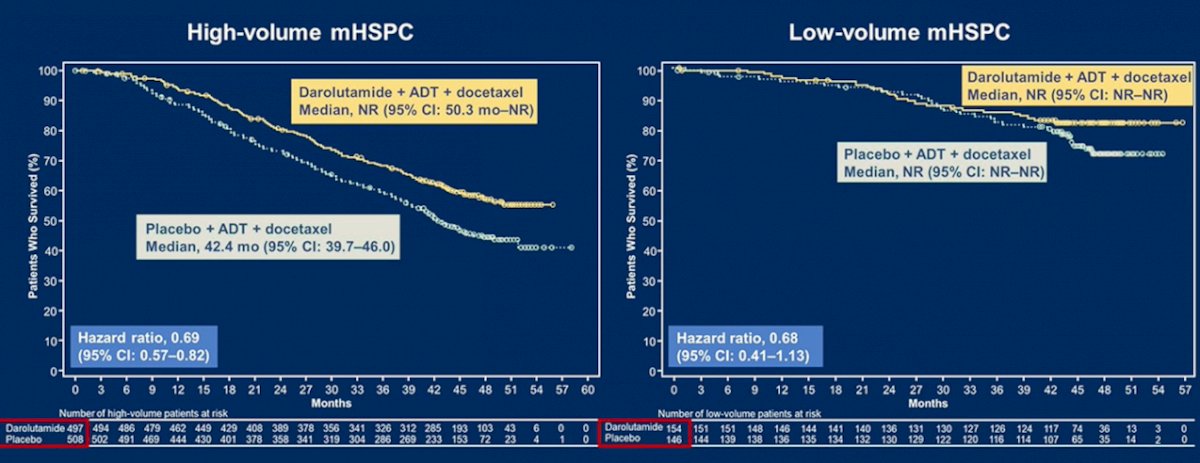
With regards to triplet therapy, the PEACE-1 trial 4 (enrolling only de novo mHSPC) showed no benefit for triplet therapy in low volume disease (median OS not reached, HR 0.93, 95% CI 0.69 to 1.28). Similarly, there was no statistically significant benefit to triplet therapy in the low volume subgroup analysis of the ARASENS trial (HR 0.68, 95% CI 0.41 to 1.13):5
With regards to treatment de-intensification, how long should patients remain on treatment? A PSA <= 0.2 ng/mL at 7 months on therapy has been suggested as a prognostic factor for longer overall survival with ADT for mHSPC, irrespective of docetaxel administration. Additionally, Dr. Tombal and Dr. Gillessen have suggested that treatment should be de-intensified after the PSA reaches < 0.2 ng/mL after 6-12 months of therapy (currently an EORTC trial proposal).
Dr. De Santis concluded her presentation with the following take-home messages:
- What systemic therapy should we use? ADT + ARPI (+ radiotherapy to the prostate in low volume mHSPC)
- For how long should we use systemic therapy?
Presented by: Maria De Santis, MD, Charite Universitaetsmedizin, Berlin, Germany
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, @zklaassen_md on Twitter during the 2023 European Association of Urology (EAU) Annual Meeting, Milan, IT, Fri, Mar 10 – Mon, Mar 13, 2023.
References
- Vale CL, Burdett S, Rydzewska LHM, et al. Addition of docetaxel or bisphosphonates to standard of care in men with localized or metastatic, hormone-sensitive prostate cancer: A systematic review and meta-analyses of aggregate data. Lancet Oncol. 2016;17(2):243-256.
- Attard G, Murphy L, Clarke NW, et al. Abiraterone acetate and prednisolone with or without enzalutamide for high-risk non-metastatic prostate cancer: A meta-analysis of primary results from two randomized controlled phase 3 trials of the STAMPEDE platform protocol. Lancet 2022 Jan 29;399(10323):447-460.
- Rush HL, Murphy L, Morgans AK, et al. Quality of life in men with prostate cancer randomly allocated to receive docetaxel or abiraterone in the STAMPEDE trial. J Clin Oncol. 2022 Mar 10;40(8):825-836.
- Fizazi K, Foulon S, Carles J, Roubaud G, et al. Abiraterone plus prednisone added to androgen deprivation therapy and docetaxel in de novo metastatic castration-sensitive prostate cancer (PEACE-1): A multicentre, open-label, randomized, phase 3 study with a 2 x 2 factorial design. Lancet. 2022 Apr 30;399(10336):1695-1707.
- Hussain M, Tombal B, Saad F, et al. Darolutamide plus androgen-deprivation therapy and docetaxel in metastatic hormone-sensitive prostate cancer by disease volume and risk subgroups in the phase III ARASENS trial. J Clin Oncol. 2023 Feb 16 [Epub ahead of print].
Alicia Morgans EAU 2023: Patient with High-Risk Localized Disease, M0 on Conventional Imaging but One Bone Lesion on PSMA PET/CT: How to Treat a Frail and Elderly Patient in this Situation
Bertrand Tombal EAU 2023: Patient with High-Risk Localized Disease, M0 on Conventional Imaging but One Bone Lesion on PSMA PET/CT: Arguments for a Surgical Approach +/- Radiotherapy +/- Systemic Treatment
Eva Comperat EAU 2023: Patient with High-Risk Localized Disease, M0 on Conventional Imaging but One Bone Lesion on PSMA PET/CT: Subtypes of Prostate Cancer and How They Change our Management
Thomas Zilli EAU 2023: Patient with High-Risk Localized Disease, M0 on Conventional Imaging but One Bone Lesion on PSMA PET/CT: Arguments for a Radiotherapeutic Approach +/- Systemic Treatment


