The performance of LND was discussed in several settings – M0 disease, M1 disease, and in recurrent retroperitoneal disease. As far back as 1990, retrospective studies were published to ascertain the role of LND in RCC, showing that 58% of the patients who underwent LND had benign nodes.1 Recent data showed that imaging remains a poor diagnostic test in the differentiation of malignant nodes, with only 42% of nodes larger than 13.7 mm being malignant.2
An important question to answer in the setting of non-metastatic RCC is to ascertain whether a durable disease-free survival can be achieved with surgical resection in patients with non-metastatic node-positive RCC. A study assessing this specific question demonstrated a 5-year disease-free survival of 39% for these patients. The favorable prognostic factors were: ECOG status 0, a single positive node, papillary histology (As opposed to clear cell), and absence of sarcomatoid features.3 Adverse prognostic features included older age, higher grade, Clear cell histology, inferior vena cava thrombus, tumor necrosis, and stage pT3/T4.4,5
Only a subset of patients with positive lymph nodes experiences a durable survival with surgery and node removal. In a study assessing these patients, with a median follow-up of 12.6 years6, it was shown that only patients with low grade and low stage disease benefited from the removal of the nodes. A more recent study, assessing retroperitoneal lymph node dissection (RPLND) in high-risk non-metastatic patients demonstrated that out of 1943 patients, 36% underwent LND, and 24% of the patients who underwent LND had positive involved lymph nodes. The median follow-up was 67.9 months with the median number of nodes being removed being 3.7 The results demonstrated no difference in overall survival between patients who underwent RPLND vs. those who didn’t.
Patient selection is extremely important when deciding when to perform LND. In a study attempting to predict which patients would have positive lymph nodes, the following were found to be predictors: Tumor size >= 10 cm, pT3-T4, Grade 3-4, sarcomatoid features, and histologic tumor necrosis.8
When assessing the templates of LND during surgery, it was seen that there is no crossover to the contralateral great vessel. In other words, there were no isolated para-aortic positive lymph nodes for right tumors, and no isolated para-caval positive lymph nodes for left-sided tumors, as can be seen in figure 1. In summary, removing all lymph nodes in non-metastatic RCC may be curative, but prospective evidence is lacking. This is supported by the European Association of Urology (EAU) and National Comprehensive Cancer Network (NCCN) guidelines stating that LND is not recommended for localized tumors without clinical evidence of lymph node invasion. When Lymph nodes are enlarged, LND can be performed for staging purposes.
Figure 1 – Lymph Node Dissection Templates:
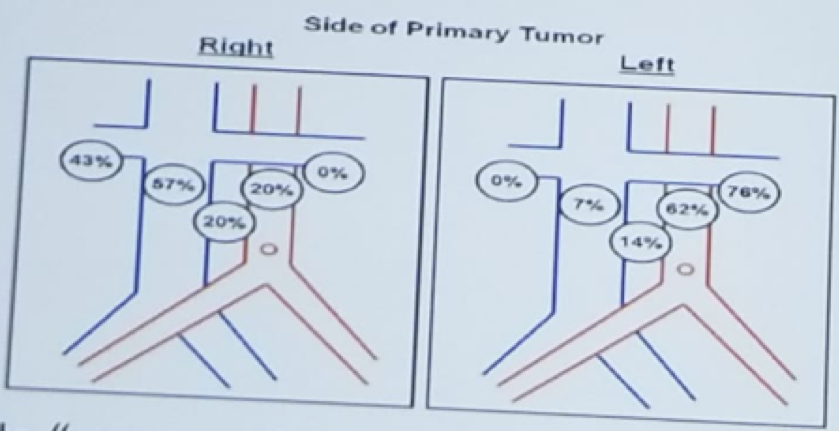
Crispen PL et al. Eur Urol 2011
Dr. Rendon moved on to discuss the role of RPLND in cytoreductive nephrectomy. The rationale for RPLND in this setting includes:
- Further cytoreduction (reduce tumor burden)
- Potential to improve response to systemic therapy
- The presence of lymphadenopathy is associated with poor response to immunotherapy.
Lastly, Dr. Rendon discussed the role of RPLND in isolated RCC recurrence. The rationale for RPLND in this setting included:
- Metastasectomy offers potentially curative treatment for metastatic RCC
- Rendering patients with a “no evidence of disease” status may delay or avoid additional recurrence.
In summary:
- In non-metastatic RCC, resection of all sites of disease (including nodal disease) may be curative. LND should be performed in very High risk N0M0 RCC, and for most N1M0 RCC.
- In metastatic RCC, cytoreduction of nodal disease may improve response to systemic therapy and reduce tumor-mediated immunological suppression, although no survival benefit was demonstrated. The current practice is to not perform LND in clinical N0M0 mRCC and to perform LND in most cN1 mRCC cases.
- In recurrent RCC – metastasectomy of retroperitoneal recurrence is associated with durable survival in many patients.
Presented by: Ricardo Rendon, MD, FRCSC, Director of Clinical Trials Chair, Research Committee
Professor, Department of Urology, Dalhousie University
Written by: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre, Twitter: @GoldbergHanan at the CUOS – Canadian Uro-Oncology Summit 2019, #CUOS19 January 10-12, 2019 Westin Harbour Castle, Toronto, Ontario, Canada
References:
1. Studer UE. Et al. J Urol 1990
2. Gershman B et al. BJU Int 2016
3. Delacroix SE et al. J Urol 2011
4. Trinh QD et al. BJU Int 2012
5. Gershman B. et al. Eur Urol 2017
6. Blom JHM et al. Eur Urol 2009
7. Ristau BT et al. J Urol 2018
8. Blute MJ et al. J Urol 2004
9. Pantuck AJ et al. J Urol 2003
10. Gershman B. et al. J Urol 2017
11. Zaid HB. et al. J Urol 2016
Further Related Content:
Lymph Node Dissection at the Time of Nephrectomy - CON


