The authors present a study aiming to describe and identify factors associated with bone-targeted therapies (BTT) usage in metastatic castrate resistant prostate cancer (mCRPC) patients in Quebec.
This retrospective study included mCRPC patients from two McGill University hospitals between January 2010 and June 2014. The cohort was divided into two groups according to mCRPC diagnosis year, with a cut-off year chose to be 2012, as it corresponded to the public reimbursement of denosumab.
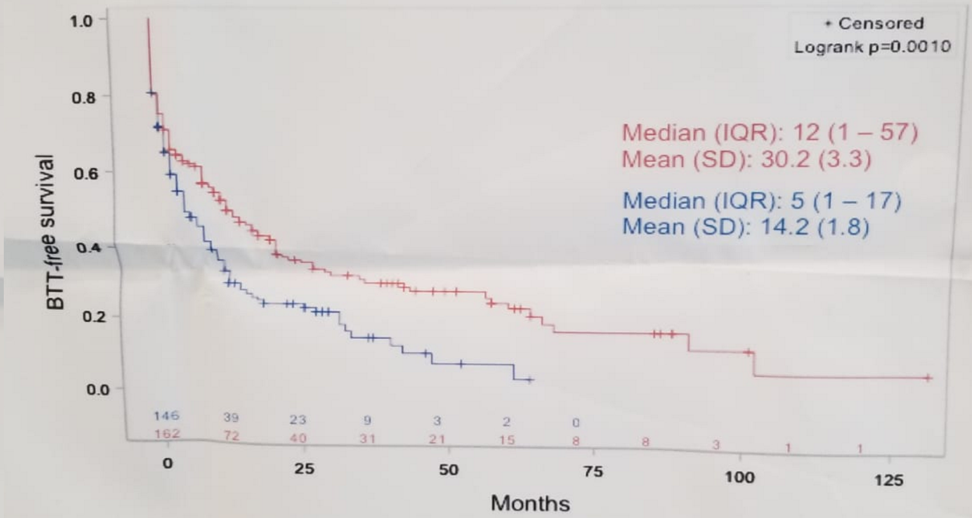
Overall, 308 mCRPC patients were assessed, of which 162 were diagnosed during 2010-2012, and 146 during 2012-2014. In the pre-2012 group, 141/162 patients (87%) had bone metastases and 80% of them had at least one prescription for a BTT (denosumab [25%], zoledronic acid [66%]). At 12 months from mCRPC diagnosis, 50% of patients received a BTT prescription in this group. In the post-2012 group, 133/146 patients (91%) had bone metastases and 84% of them had at least one prescription for a BTT (denosumab [89%], zoledronic acid [8.9%]). Figure 1 demonstrates Kaplan Meier curve of time to first BTT prescription since MCRCP diagnosis before 2012 and after 2012. In a multivariable model, the predictors of BTT usage included:
- Bone metastases (hazard ratio [HR] 4.4; 95% confidence interval [CI] 2.6-7.5)
- Visceral metastases (HR 3.6; 95% CI 1.8-7.2) at CRPC diagnosis,
- Symptomatic disease at mCRPC ƒdiagnosis (HR 1.5; 95% CI 1.1-2.1),
- mCRPC diagnosis post-2012 (HR 1.5; 95% CI 1.1-2.0).
The authors concluded that BTT use became more frequent after the introduction of Denosumab for the management of MCRPC patients in 2012 in Quebec. Bone metastases, symptoms and 1 year of MCRPC diagnosis were strong predictive factors for BTT usage. These results provide real-world data on usage of novel therapies in MCRPC management.
Figure 1 – Kaplan-Meier of time to first BTT prescription since MCRPC diagnosis:
Presented by: Alice Dragomir, MD, Assistant Professor, McGill University — Montreal, Canada
Co-Authors: Halima Lahcene1,2, Marie Vanhuyse3,4, Jason Hu1,2, Franck Bladou1,4, Wassim (Wes) Kassouf1,4, Fabio Cury4,5, Jonathan Primiani1,2, Sara Nazha1,2, Armen Aprikian1,4.
Author Information:
1. Division of Urology, McGill University, Montreal, QC, Canada
2. Research Institute of the McGill University Health Centre, McGill University, Montreal, QC, Canada
3. Division of Medical Oncology, McGill University, Montreal, QC, Canada
4. McGill University Health Centre, McGill University, Montreal, QC, Canada
5. Division of Radiation Oncology, McGill University, Montreal, QC, Canada
Written By: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre, @GoldbergHanan at the 73rd Canadian Urological Association Annual Meeting - June 23 - 26, 2018 - Halifax, Nova Scotia


