The single-arm SAUL study enrolled a broader patient population, including patients with poor clinical characteristics and/or immune-mediated conditions, more representative of real-world practice than typically enrolled in randomized phase III immunotherapy trials. Patients with urinary tract carcinoma received atezolizumab 1200 mg q3weeks until disease progression/unacceptable toxicity. Baseline characteristics, safety and efficacy were analyzed in subgroups of patients with upper tract urothelial carcinoma (subdivided into renal pelvis or ureter UC) versus bladder urothelial carcinoma.
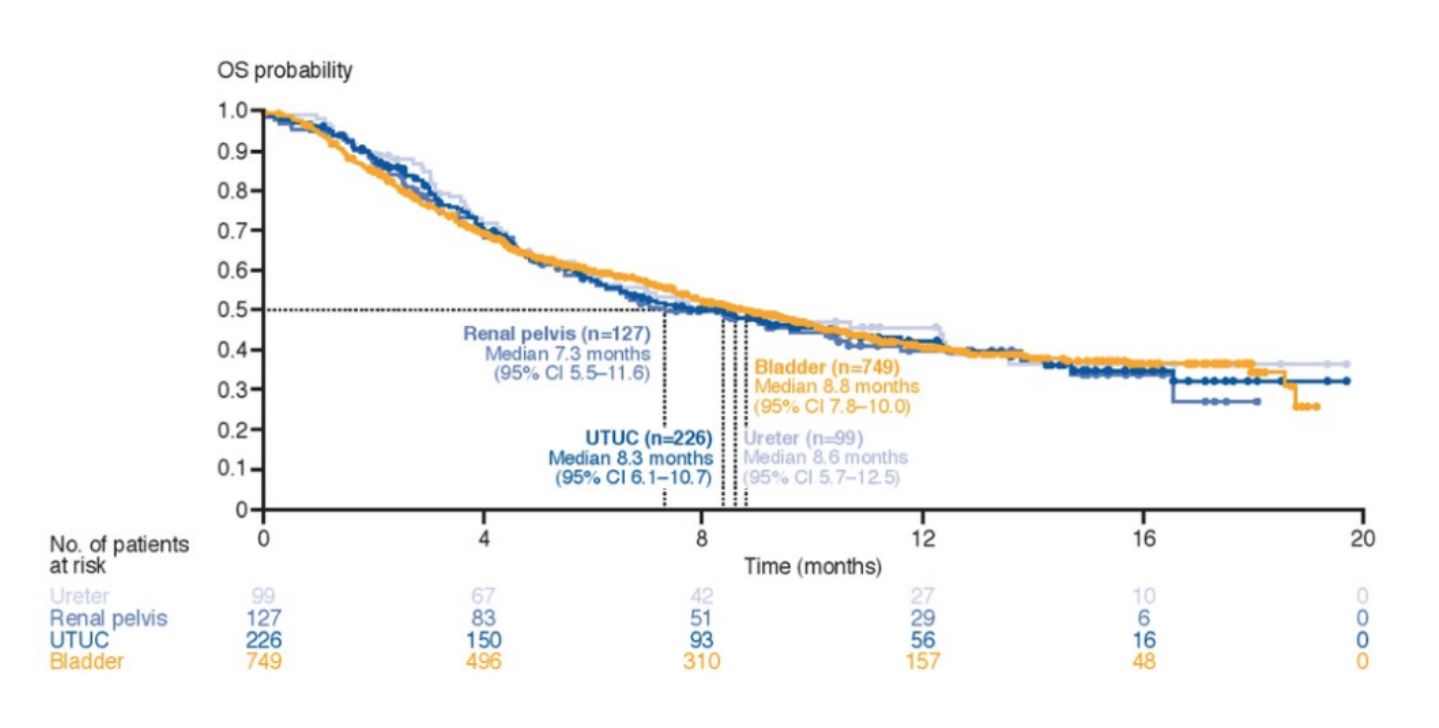
The upper tract urothelial carcinoma group included 226 patients of which 99 had ureteral carcinoma and 127 had renal pelvic carcinoma. Median follow-up was 13.2 months in the upper tract urothelial carcinoma group and 12.2 months in the bladder urothelial carcinoma group. Baseline characteristics in the subgroups were generally similar, however, patients with upper tract urothelial carcinoma had fewer patients that had 0 prior lines of therapy for metastatic disease compared to bladder urothelial groups (30% vs 41%). Second, upper tract urothelial carcinoma patients had a smaller proportion of patients reporting as current/former smokers (59% vs 70%). Urinary tract infection was more common in bladder cancer patients than upper tract urothelial carcinoma patients (17% vs 11%), and arthralgia was more common in patients with ureter than pelvis carcinoma (12% vs 6%). As of the data cutoff date (September 16, 2018), deaths had been recorded in ~55% of patients in each of the subgroups, mostly from disease progression. The OS by tumor location is comparable between the subgroups and is highlighted as follows:
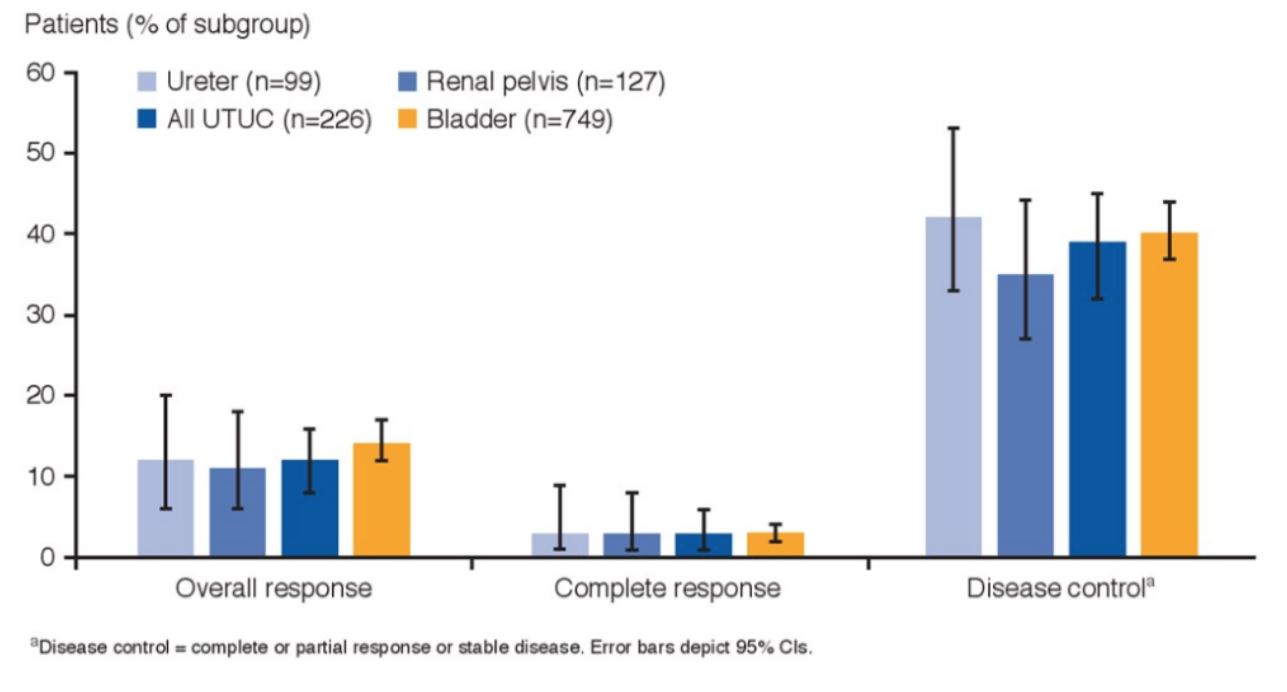
Additionally, overall response, complete response and disease control were comparable between all four groups:
Dr. Sternberg concluded her presentation assessing subgroup analyses among patients receiving atezolizumab as part of the SAUL study with the following take-home messages:
- These exploratory analyses of SAUL showed very similar efficacy and safety in upper tract vs bladder urothelial carcinoma
- This provides reassurance that atezolizumab is active and has an acceptable safety profile in patients with upper tract urothelial carcinoma, who are generally expected to have worse outcomes than bladder urothelial carcinoma patients
- Final results are expected 4 years after enrollment of the last patient
Presented by: Cora Sternberg, MD, Weill Cornell Medical School, New York, New York
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the 2020 Genitourinary Cancers Symposium, ASCO GU #GU20, February 13-15, 2020, San Francisco, California
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the 2020 Genitourinary Cancers Symposium, ASCO GU #GU20, February 13-15, 2020, San Francisco, California
References:
1. Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: A single-arm, multicentre, phase 2 trial. Lancet 2016;387(10031):1909-1920.
2. Powles T, Duran I, van der Heijden MS, et al. Atezolizumab versus chemotherapy in patients with platinum-treated locally advanced or metastatic urothelial carcinoma (IMvigor211): A multicentre, open-label, phase 3 randomized controlled trial. Lancet 2018;391:748-757.
3. Sternberg CN, Loriot Y, James N, et al. Primary results from SAUL, a multinational single-arm safety study of atezolizumab therapy for locally advanced or metastatic urothelial or nonurothelial carcinoma of the urinary tract. Eur Urol 2019 Jul;76(1):73-81.


