San Francisco, CA (UroToday.com) As part of the session on confronting obstacles in the treatment of nonmuscle invasive and upper tract urothelial carcinoma at GU ASCO 2020, Jean Hoffman-Censits, MD, discussed treatment options for upper tract urothelial carcinoma. Upper tract disease accounts for 5-10% of all urothelial cancers, with a median age of 69-73 years. Recurrence rates are up to 50% and may be extraluminal, intraluminal or in the contralateral upper tract. Currently, the standard of care, regardless of disease grade is radical nephroureterectomy.
As presented by Seth Lerner, MD, at AUA 2019, Dr. Hoffman-Censits discussed the results of the Olympus Trial, which was nephron-sparing management of low-grade upper tract urothelial carcinoma with UGN-101 (mitomycin gel) for instillation. UGN-101 is a poloxamer 407 based inverse thermosensitivity able to deliver mitomycin C (4mg/1ml gel) to the upper tracts. It is a solid at body temperature and a liquid at lower temperatures. This was a prospective, phase 3, open label, single-arm trial in patients with low grade disease that were given six weekly treatments of UGN-101. The primary objective was to evaluate safety and tumor ablative effects of UGN-101 at the primary disease evaluation visit. A complete response was comprised of a negative ureteroscopy and cytology +/- biopsy. As follows is the full study design of Olympus:

There were 71 patients treated and evaluated with a complete response rate of 59%, and 27 of 41 patients have undergoing six-month surveillance with 89% of these patients remaining disease free. Among 34/71 patients that were endoscopically unresectable, 59% of these patients achieved a complete response and 17/20 (85%) were disease free at six-month follow-up. There are several other intraluminal trials ongoing:
- Treatment of tumors in the urinary collecting system of the kidney or ureter using a light activated drug (WST11). The PI is Jonathan Coleman, MD, and 14/18 patients have been treated and there are plans for a multi-center trial
- Phase I study of percutaneous valrubicin for upper tract urothelial carcinoma. The PI is Wade Sexton, MD, and enrolment is ongoing
To summarize low grade upper tract urothelial carcinoma, Dr. Hoffman-Censits offered the following summary points:
- Radical nephroureterectomy is the gold standard, but the last resort.
- Endoscopic management is an option for well-selected patients with low grade upper tract urothelial carcinoma, particularly those with low-grade/low-risk disease.
- Intracavitary therapies have a limited role in low risk/low grade disease.
- Chemoablative technologies offer promising preliminary data.
- A better understanding of endoscopic options by urologists can lead to better diagnosis and therapeutic strategies for upper tract urothelial carcinoma.
Among patients with high-grade upper tract urothelial carcinoma that cisplatin eligible, cisplatin eligibility declines from 58% preoperatively to 15% postoperatively. Four prospective trials have looked at neoadjuvant chemotherapy for upper tract urothelial carcinoma and are summarized as follows:
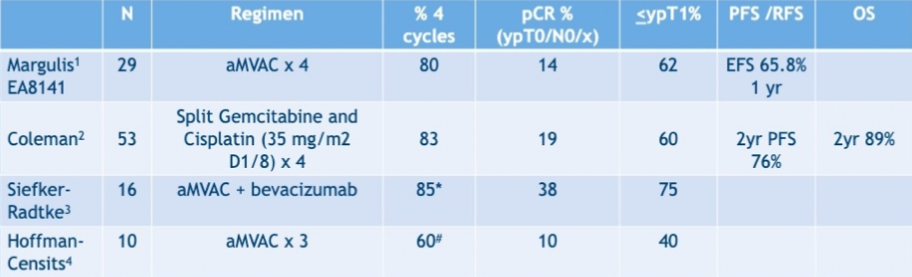
There are several ongoing neoadjuvant chemotherapy trials on going:
- Neoadjuvant chemotherapy versus surgery alone in patients with high-grade upper tract urothelial carcinoma: cisplatin + gemcitabine (in China)
- Phase II study of gemcitabine/cisplatin chemotherapy combined with PD-1 inhibitor (toripalimab) in the neoadjuvant treatment of upper urinary and muscle invasive urothelial carcinoma (in China)
- Feasibility of neoadjuvant versus adjuvant chemotherapy in upper tract urothelial carcinoma (URANUS; in Europe)
Dr. Hoffman-Censits notes that clinical staging of upper tract urothelial carcinoma remains a challenge, and the 5-year CSS rate for high-grade upper tract urothelial carcinoma is only 57%. However, the pathologic complete response rates are lower than bladder urothelial neoadjuvant trials, and down-staging with chemotherapy appears to correlate with improved survival in multiple upper tract urothelial carcinoma studies.
Two years ago at GU ASCO, Alison Birtle, MD, presented the exciting results of the POUT trial. This trial addressed whether adjuvant chemotherapy improves disease free survival (DFS) for patients with histologically confirmed pT2-T4 N0-3 M0 upper tract urothelial carcinoma. There were 248 patients that were ≤90 days post nephroureterectomy who were randomized (1:1) to 4 cycles of gemcitabine-cisplatin (gemcitabine-carboplatin if GFR 30-49ml/min) or surveillance with chemotherapy given on recurrence, if required. The primary endpoint was DFS, and patients had cross-sectional imaging and cystoscopy every 6 months for the first 2 years, then annually to 5 years. At the time of interim analysis, median follow-up was 17.6 months (IQR 7.5-33.6). Patients were a median 69 years of age (range 36-88), 30% had pT2 disease, 65% pT3, and 91% pN0. There were 47 (38.2%) DFS events in the surveillance cohort and 29 (23.2%) in the chemotherapy cohort; the unadjusted HR was 0.47 (95%CI 0.29-0.74) in favor of chemotherapy (log-rank p= 0.0009). Two-year DFS was 51% for surveillance (95%CI 39-61) and 70% for chemotherapy (95%CI 58-79). Metastasis-free survival also favored chemotherapy, with a HR of 0.49 (95%CI 0.30-0.79, p=0.003).
There are currently three ongoing phase III adjuvant immunotherapy studies for upper tract urothelial carcinoma/bladder cancer ongoing as summarized in this table:

In the upper tract urothelial carcinoma population, we are often in the situation where patients either have a solitary kidney or have chronic kidney disease with two kidneys. According to Surena Matin, MD, these patients are the “trial ineligible, guideline ignored” patients. In a retrospective study among patients with a solitary kidney, the 5-year CSS rates for patients with eGFR ≥ 60 mL/min was 92.9%, for those with 15 ml/min ≤ eGFR < 60 mL/min was 75.3% and for patients with eGFR <15 mL/min was 63.7%. The 5-year OS and recurrence-free survival rates were 92.9 and 53 % for patients with eGFR ≥ 60 mL/min, respectively, 75.3 and 64.8 % for patients with 15 ml/min ≤ eGFR < 60 mL/min, respectively, and 63.7 and 29.5 % for patients with eGFR <15 mL/min, respectively. For these patients, there is currently a clinical trial ongoing assessing pembrolizumab in combination with BCG after ablation in patients with upper tract urothelial carcinoma that have not/will not undergo a nephroureterectomy.
Dr. Hoffman-Censits concluded her upper tract urothelial carcinoma presentation for local and systematic therapy with several take-home points:
- For high-grade disease, multidisciplinary management is increasingly important
- Evaluation for perioperative chemotherapy should be considered as standard of care
- There is more data emerging that perioperative chemotherapy improves survival outcomes
- The neoadjuvant setting may be the only opportunity to deliver cisplatin and neoadjuvant chemotherapy appears to be underutilized
- Upper tract urothelial carcinoma in a solitary kidney is an unmet need where novel endpoints should be considered
Presented by: Jean Hoffman-Censits, MD, Assistant Professor of Oncology, The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, MD
Written by: Zachary Klaassen, MD, MSc – Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md, at the 2020 Genitourinary Cancers Symposium, ASCO GU #GU20, February 13-15, 2020, San Francisco, California.
References:
- Su X, Fang D, Zhang L, et al. Treatment strategies for upper tract urothelial carcinoma (UTUC) of a solitary kidney: A single-institutional analysis of 61 cases. Int Urol Nephrol 2016 Oct;48(10):1601-1608.


