The presented study design (the OLYMPUS study) is presented in figure 1. This was a single arm prospective phase 3 open-label study in patients with LG UTUC. The primary objective was to evaluate the safety and tumor ablative effect of UGN-101. All patients received six once-weekly instillations of UGN-101 with a mitomycin dosage of 4 mg/1ml of TC-3 gel. The target population was 74 patients with pathologically confirmed LG non-invasive UTUC. The trial was designed with a 90% power to demonstrate that the observed complete response rate is above 15%.
Figure 1 – OLYMPUS study design:

The inclusion criteria included new or recurrent LG UTUC with biopsy confirmed LG and cytology negative for high grade (HG) disease. Patients needed to have at least 1 papillary LG tumor (0.5-1.5 cm in diameter), and the tumor needed to be located superior to the ureteropelvic junction (UPJ). The full inclusion and exclusion criteria are shown in figure 2.
Figure 2- OLYMPUS trial inclusion and exclusion criteria:

The final cohort included 71 patients with their baseline data shown in table 1. The results demonstrated that out of the 71 patients, a complete response rate of 59% was witnessed. A total of 66% had undergone six months of surveillance, and 89% remained disease free at six months of follow-up. A total of 48% were deemed to be endoscopically unresectable, with 59% of them achieving a complete response, and 85% remaining disease free at six months of follow-up.
Table 1 – Demographic data:

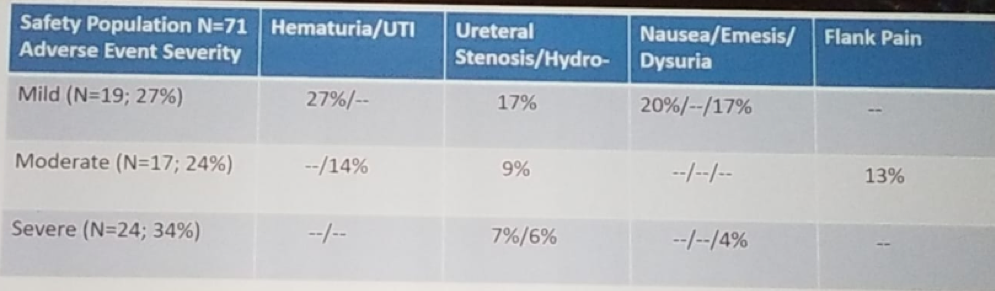
When considering the adverse events (Table 2), 2.8% had life-threatening events, and 4.2% were fatal (stroke one patient, failure to thrive one patient, and sudden death one patient). It is noteworthy that all life-threatening and fatal events were not related to UGN-101 or the procedure.
Table 2- Adverse events:
Dr. Lerner concluded by stating that data from the OLYMPUS trial suggest that UGN-101 can ablate LG tumors with a high initial complete response rate, that appears durable, with an ongoing follow-up. The high rate of initial disease eradication obviated the need for kidney removal in 48% of patients deemed to have unresectable disease. Lastly, UGN-101 is an investigational drug that if approved by the FDA, may provide an alternative for the initial management of patients with LG UTUC.
Presented by: Seth Lerner, MD, FACS, Baylor College of Medicine
Written By: Hanan Goldberg, MD, Urologic Oncology Fellow (SUO), University of Toronto, Princess Margaret Cancer Centre @GoldbergHanan at American Urological Association's 2019 Annual Meeting (AUA 2019), May 3 – 6, 2019 in Chicago, Illinois


