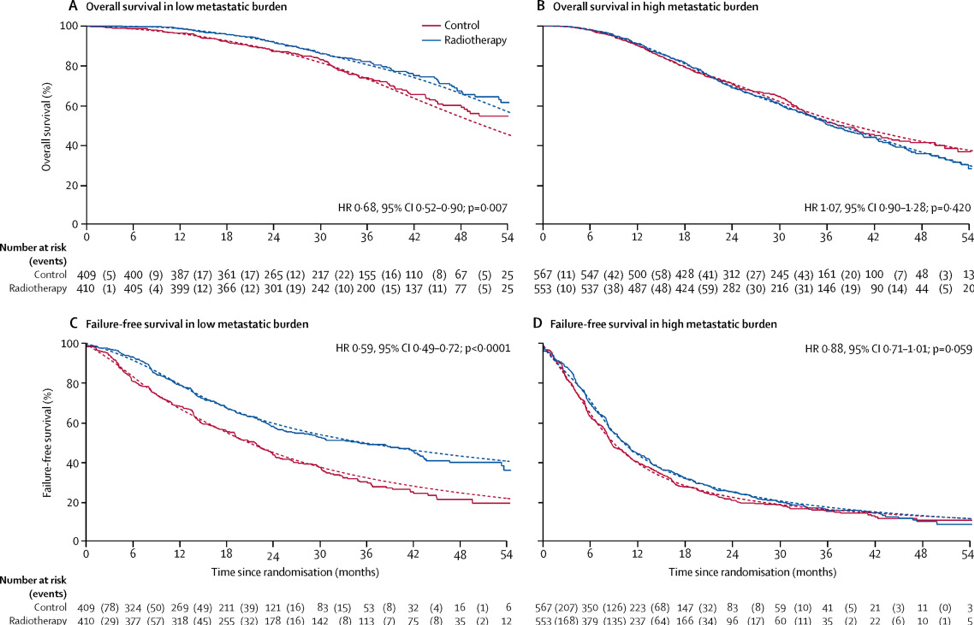
The first case presented by Dr. Sandler was of a 65-year-old male with an initial PSA of 18 ng/ml. A mpMRI showed a T3a lesion in the right peripheral zone and a subsequent biopsy of this lesion demonstrated Gleason 4+4 disease. Conventional imaging was negative for metastatic disease, however, a PSMA PET demonstrated oligometastatic disease (3 small skeletal lesions). Dr. James started the discussion by highlighting that radiotherapy is a standard of care in M1 prostate cancer. Data from the STAMPEDE trial has assessed the benefit of radiotherapy to the primary tumor among me with metastatic disease.1 In the analysis by metastatic burden, overall survival was improved in patients with low metastatic burden at baseline who were allocated radiotherapy (HR 0.68, 95% CI 0.52–0.90; 3-year survival 73% with control vs 81% with radiotherapy). However, there was evidence of heterogeneity of treatment effect by metastatic burden (interaction p=0.0098), whereby patients with a high metastatic burden had no evidence of a treatment effect (HR 1.07, 95% CI 0.90–1.28):
The panel then polled the audience with the following question and answer choices (and audience answers): For men presenting with 1-3 metastases and no prior therapy my preferred therapy is:
- ADT only (7%)
- ADT + docetaxel (3.5%)
- ADT + docetaxel + treatment of the primary (11.4%)
- ADT + novel AR targeting (15.8%)
- ADT + novel AR targeting + treatment of the primary (62.2%)
Based on limited retrospective data in this setting, the panel then polled the audience again with the following question: For men presenting with 1-3 metastases and no prior therapy my preferred therapy is (systemic therapy = ADT +/- docetaxel/ART):
- Systemic therapy only (4.8%)
- Systemic therapy + radiotherapy to the prostate (47.6%)
- Systemic therapy + radiotherapy to the prostate and lymph nodes (44.4%)
- Systemic therapy + surgery (3.2%)
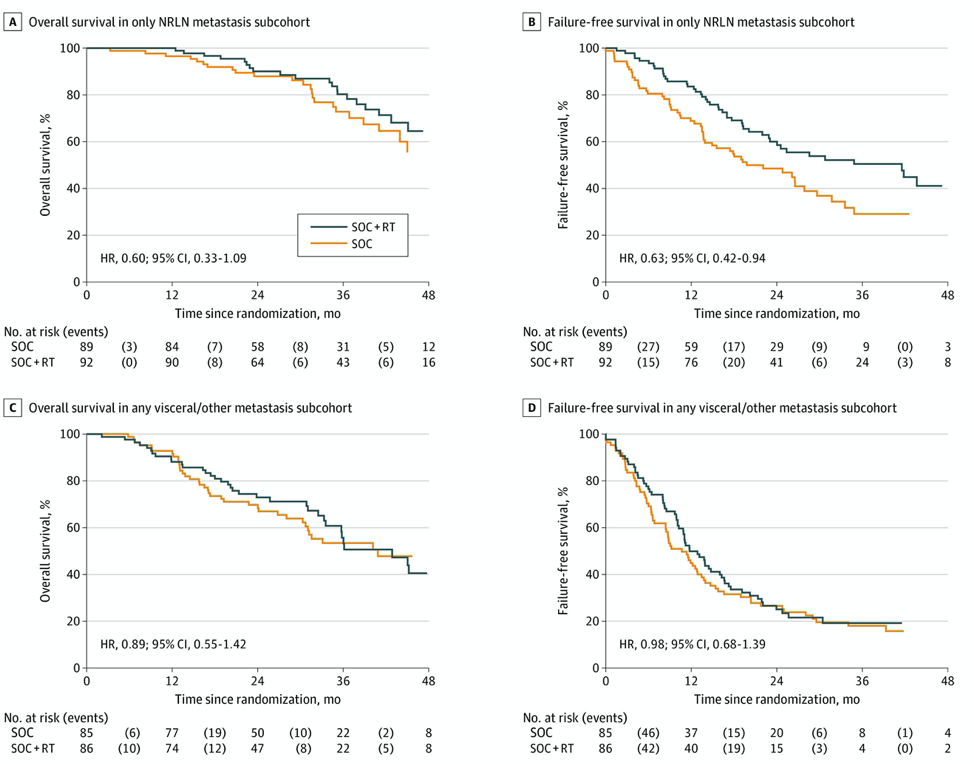
Dr. James then discussed new data from the STAMPEDE trial recently published in JAMA Oncology.2 Among 181 patients with only NRLN metastasis (M1a), there was a strong indication of survival benefit from prostate RT (HR 0.60, 95% CI 0.33-1.09), with an absolute improvement in 3-year survival with prostate RT of 7% (73% standard of care; 80% standard of care + radiotherapy). There was also good evidence of improvement in failure-free survival from prostate radiotherapy with only NRLN metastasis (HR 0.63, 95% CI 0.42-0.94). A similar analysis in patients with any visceral/other metastasis showed no evidence of benefit from adding prostate radiotherapy on OS or failure-free survival (OS HR 0.89, 95% CI 0.55-1.42; failure-free survival HR 0.98, 95% CI, 0.68-1.39):
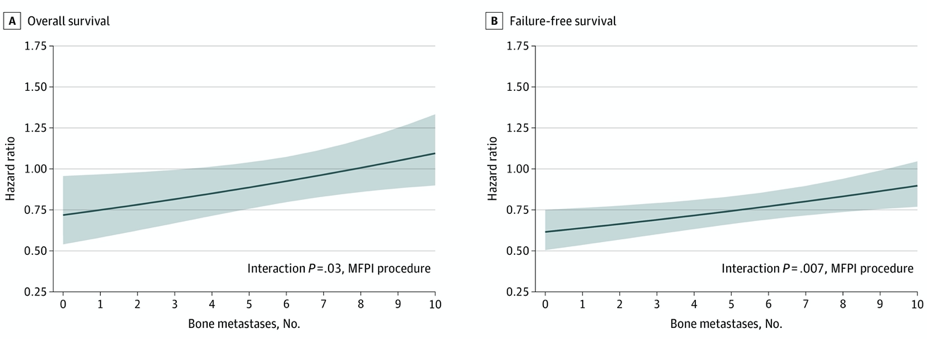
Additionally, there was evidence of survival benefit with the addition of prostate radiotherapy to up to 3 bone metastases, with the upper 95% CI crossing the line of equivalence (HR 1) just after this. Similarly, for failure-free survival, there was good evidence of a treatment interaction with bone metastasis count, with the upper 95% CI crossing the line of equivalence at around 9 bone metastases:
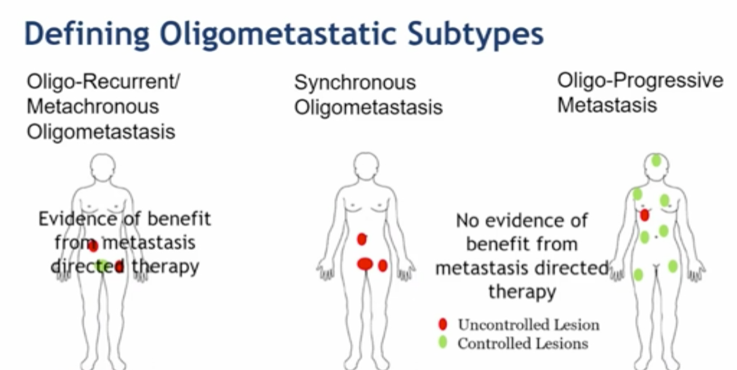
Dr. James notes that it is important to define oligometastatic subtypes, which may be classified as (i) oligo-recurrent/metachronous oligometastasis, (ii) synchronous oligometastasis, and (iii) oligo-progressive metastasis.
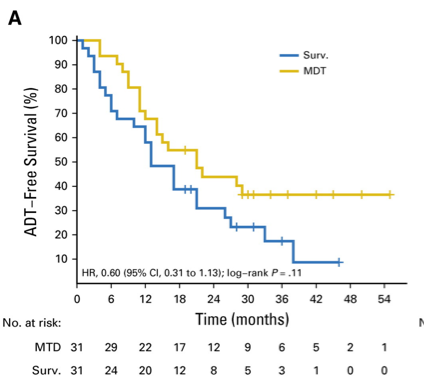
There have been two phase II trials assessing metastasis-directed therapy for oligometastatic prostate cancer. In the STOMP trial, Ost and colleagues3 randomly assigned 62 patients to either surveillance or metastasis-directed therapy of all detected lesions (surgery or stereotactic body radiotherapy), with a primary endpoint of ADT-free survival. At a median follow-up time of 3 years (IQR 2.3-3.75 years), the median ADT-free survival was 13 months (80% CI 12 to 17 months) for the surveillance group and 21 months (80% CI 14 to 29 months) for the metastasis-directed therapy group (HR 0.60, 80% CI 0.40 to 0.90; log-rank p = 0.11):
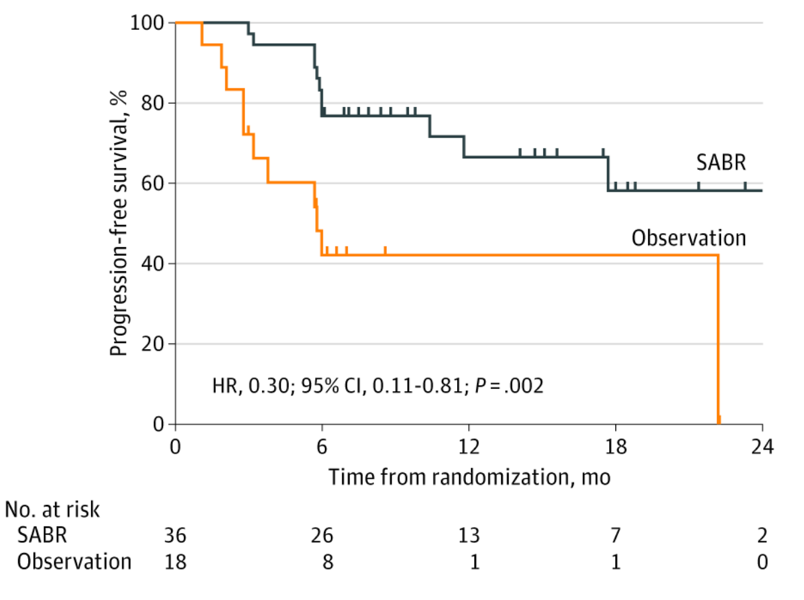
The second phase II trial published recently was the ORIOLE trial,4 randomizing 54 men in a 2:1 ratio to receive stereotactic body radiotherapy or observation. The primary endpoint for this trial was progression at 6 months, defined as a PSA increase, radiographic or symptomatic progression, ADT initiation, or death. Progression at 6 months occurred in 7 of 36 patients (19%) receiving stereotactic body radiotherapy and 11 of 18 patients (61%) undergoing observation (p = 0.005). Furthermore, treatment with stereotactic body radiotherapy improved median progression-free survival (not reached vs 5.8 months; HR 0.30, 95% CI 0.11-0.81; p = 0.002):
For those patients in the stereotactic body radiotherapy arm that had a PSMA PET-CT scan, the proportion of men with disease progression at 6 months was 5% in those who did not have any untreated lesions, compared to 38% in those who did have some untreated PSMA avid lesions (p=0.03).
Dr. Sandler then presented the second case for discussion, which was a 66-year-old male who underwent a robotic prostatectomy with standard pelvic lymph node dissection for pT2cN0M0R0 Gleason 4+4 disease and an initial post-op PSA 0.04 ng/mL with a rise to 1.0 over the next year. CT and bone scans were negative for metastatic disease, however, sodium-fluoride PET scan showed a 9th rib metastasis. Treatment was initiated with 3,000 cGy to the rib metastasis with a short course of ADT. The PSA was undetectable initially but then rose to 1.6 ng/mL two years later. At that time, a fluciclovine PET scan was obtained that showed only one area of concern for metastasis at the left pelvic lymph node chain.
The panel then offered the following question to the audience for response: Treatment decision – how would you proceed?
- ADT alone (0.5%)
- Salvage lymph node dissection with or without a course of ADT (5.1%)
- Salvage radiation therapy with or without a course of ADT (72.7%)
- ADT with the addition of the androgen receptor inhibitor abiraterone (21.7%)
Dr. Given notes that salvage lymphadenectomy for nodal recurrence is generating increasing interest with improvements in PET imaging identifying oligometastatic disease. The attraction is the potential to limit or delay ADT, given the known morbidity of hormone therapy. The extent of node dissection has been variable and needs to be elucidated, for example, whether we should do pelvic nodes only +/- presacral +/- retroperitoneal lymph nodes. Salvage lymph node dissection has a generally reasonable morbidity profile, with the most common complication being ileus, DVT, and lymphoceles. Across the studies, the Clavien Grade 3 complication rate is 0-27% and this procedure can be performed laparoscopic, open, or robotic.
In a systematic review assessing the benefit of salvage lymphadenectomy, Pisano et al.5 found that salvage lymphadenectomy is still characterized by a lack of standardization in patient selection and surgical template. The primary objectives of this procedure are to prolong progression-free survival and to delay the need for systemic therapy. The improvements in preoperative imaging techniques in conjunction with the wide use of minimally invasive surgery have generated growing interest in this procedure. As follows is a summary of the main results of studies that have reported on salvage lymph node dissection in patients with nodal recurrent prostate cancer for which 5-year follow-up data are available:
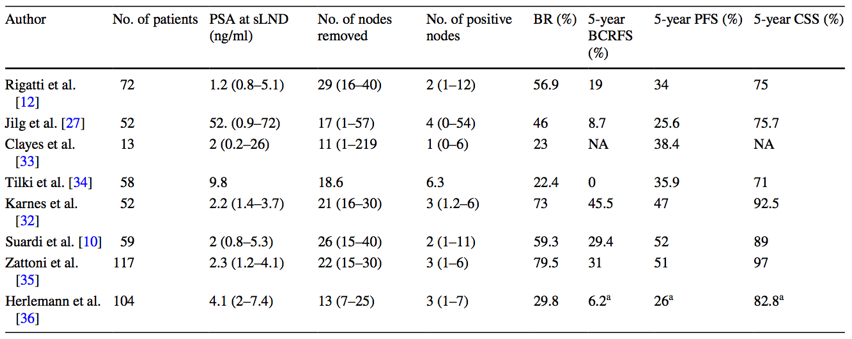
With regards to salvage lymph node dissection in recurrent castrate-sensitive oligometastatic disease, the 5-year biochemical recurrence-free survival rates range from 0-45%. Salvage lymph node dissection may prolong survival and certainly can delay the need for ADT in carefully selected patients. Indeed, patient selection is key, namely PSA <4 ng/mL and absence of retroperitoneal disease. Dr. Given notes that salvage lymph node dissection for nodal recurrence remains controversial with limited small studies showing potential benefit with tolerable morbidity, but no published randomized trials completed.
Going back to the second case, the patient was treated with 3500 cGy to the pelvis and a 2000 cGy boost to the left pelvic lymph nodes without ADT. There was an initial good PSA response with a subsequent rise in PSA to 2 ng/mL with fluciclovine PET showing activity in a lymph node just below the aortic bifurcation. The panel then polled the audience with the following question: What should the next treatment be?
- Salvage lymph node dissection? (4.2%)
- Radiation therapy to the lymph node? (26.7%)
- ADT with or without ARI or abiraterone? (45.5%)
- Combination of all of the above? (23.6%)
Dr. Sandler then discussed the third case, which was a 66-year-old male with cT2cN1M0, Gleason 5+4 (12/12 cores positive), with a PSA of 14 ng/mL. The patient was subsequently treated with 80 Gy of IG-IMRT and long-term androgen deprivation therapy, with the PSA ranging from 0.7-1.3 during the follow-up period. At 2-years of follow-up, the PSA was 5.4 with testosterone <20 ng/dL and new bulky >2.5 cm left para-aortic and retroperitoneal lymph nodes. The panel then asked the audience what the next line of therapy should be?
- Sipuleucel-T (8.3%)
- Abiraterone (45.9%)
- Docetaxel (7.7%)
- Enzalutamide (30.9%)
- Lutecium-177 radioligand therapy (7.2%)
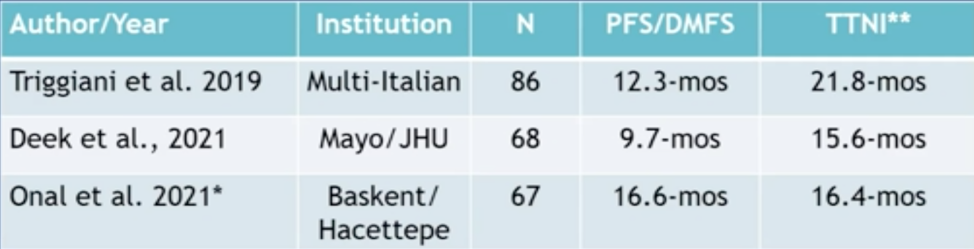
This patient ultimately underwent stereotactic ablative therapy (5.6 Gy x 5 QOD) with a PSA nadir of 0.14 ng/mL and a decrease in the size of the retroperitoneal lymph nodes. Dr. Tran notes that the clinical data for metastasis-directed therapy in oligometastatic CRPC is small with predominantly retrospective studies of <100 patients. Time to next intervention in these studies ranged from 15.6 to 21.8 months:
However, this is a very hot area of research and there are several ongoing clinical trials for metastasis-directed therapy among patients with oligometastatic CRPC, highlighted in the following table:
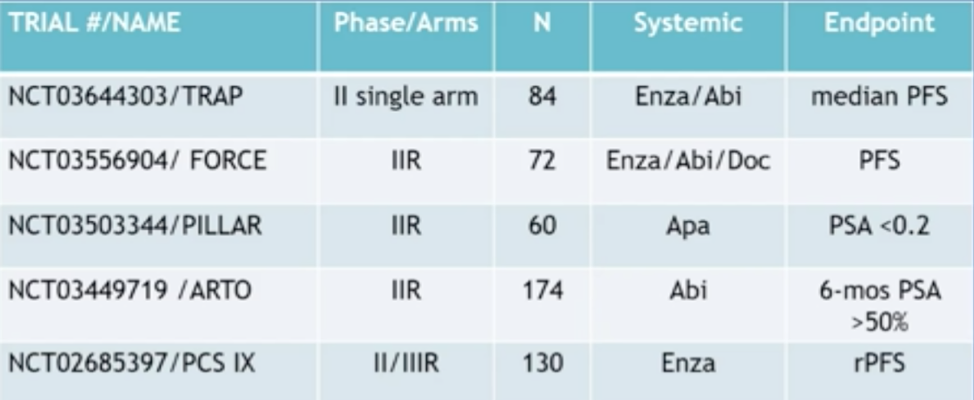
In Dr. Tran’s opinion, metastasis-directed therapy is defined by the treatment of 5 or fewer lesions, although this definitely is quite variable. Dr. James concluded that the STAMPEDE platform is also delving into metastasis-directed therapy, randomizing patients to treatment of oligometastatic disease, as well as assessing the imaging modality received and the impact on patient outcomes.
Chair: Howard M. Sandler, MD, Cedars-Sinai Medical Center, Los Angeles, CA
Radiation Oncologist’s Perspective: Phuoc T. Tran, MD, PhD, Johns Hopkins University School of Medicine, Baltimore, MD
International Perspective: Nicholas D. James, MBBS, PhD, The Institute of Cancer Research, London, UK
Community Perspective: Robert Given, MD, Eastern Virginia Medical School, Norfolk, VA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021
References:
- Parker CC, James ND, Brawley CD, et al. Radiotherapy to the primary tumour for newly diagnosed, metastatic prostate cancer (STAMPEDE): A randomized controlled phase 3 trial. Lancet 2018 Dec 1;392(10162):2353-2366.
- Ali A, Hoyle A, Haran AM, et al. Association of bone metastatic burden with survival benefit from prostate radiotherapy in patients with newly diagnosed metastatic prostate cancer: A secondary analysis of a randomized clinical trial. JAMA Oncol. 2021 Apr 1;7(4):555-563.
- Ost P, Reynders D, Decaestecker K, et al. Surveillance of Metastasis-Directed Therapy for Oligometastatic Prostate Cancer Recurrence: A Prospective, Randomized, Multicenter Phase II Trial. J Clin Oncol 2018 Feb 10;36(5):446-453.
- Phillips R, Shi WY, Deek M, et al. Outcomes of Observation vs Stereotactic Ablative Radiation for Oligometastatic Prostate Cancer: The ORIOLE Phase 2 Randomized Clinical Trial. JAMA Oncol 2020 Mar 26;6(5):650-659.
- Pisano F, Gaya JM, Breda A, et al. Salvage lymphadenectomy in recurrent prostate cancer: Is there evidence of real benefit? World J Urol. 2019 Aug;37(8)?1551-1556.


