Dr. McKay notes that renal cell carcinoma is common, being the 6th most common cancer in males (5% of new cancer cases per year) and the 9th most common in females (3% of new cancer cases per year). For localized disease, there is certainly increased risk of disease recurrence by T stage, and translating to substantially worse 5-year survival when stratified by overall stage:
- Stage 1: 95%
- Stage 2: 88%
- Stage 3: 59%
- Stage 4: 20%
Additional factors are also predictive of recurrence and have been highlighted in predictive models, including grade, performance status, size, tumor necrosis, histology, age, and gender:
Dr. McKay notes that the term ‘adjuvant’ is derived from the Latin term adjuvare, meaning ‘to help’ and first coined by Dr. Paul Corbone at the NCI in 1963. Investigators reasoned that adjuvant chemotherapy could eliminate “cells dislodged into the blood and lymph during surgical manipulation”. In 1968, the NSABP published its B-01 trial results for the first randomized trial that evaluated the effect of an adjuvant alkylating agent in breast cancer. Ultimately, the goal of adjuvant therapy is to improve cure rates, risk of recurrence, and overall survival.
Dr. McKay highlights that the treatment options have expanded greatly over the last several decades, as highlighted in the following figure: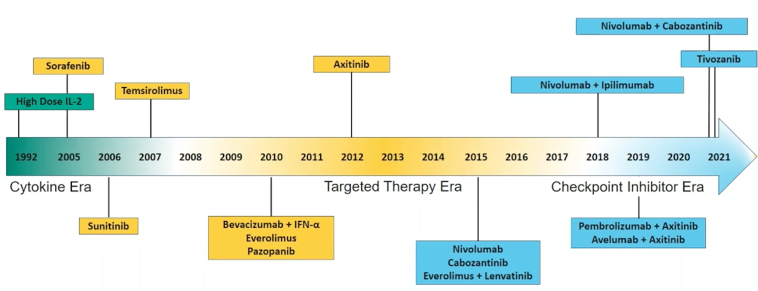
With regards to the history of adjuvant therapy for RCC, there were several trials (from 1992 through 2014) testing different cytokine therapies that did not improve survival. More recently, adjuvant targeted therapy trials emerged with mixed results. These trials included the ASSURE trial1, the S-TRAC trial2, the PROTECT trial3, the ATLAS trial4, and the SORCE trial5. There were no OS benefits from these trials, however, the S-TRAC trial showed a DFS benefit (HR 0.76, 95% CI 0.59-0.98):
Dr. McKay notes that neither prognostic category nor dose intensity altered a lack of DFS benefit in the ASSURE trial. Alternatively, the S-TRAC study of adjuvant sunitinib was positive for DFS but with absolutely no signal for a survival benefit (HR 0.92, 95% CI 0.66-1.28). However, the DFS benefit associated with adjuvant sunitinib in S-TRAC came at a high cost, as it was associated with increased toxicity and lower quality of life scores:
The FDA approved sunitinib for the adjuvant treatment of adult patients at high risk of recurrent RCC following nephrectomy in the US on November 16, 2017, however, sunitinib is not approved for use in this setting by the EMA.
Early studies of immunotherapy demonstrated that single-agent checkpoint inhibitor therapy has efficacy in advanced RCC. In patients pre-treated with VEGF inhibitors, the CheckMate 025 phase 3 trial randomized patients to nivolumab versus everolimus, noting a survival benefit for nivolumab (25.8 versus 19.7 months) and hazard ratio of 0.73 (95% CI 0.62-0.85)6. In treatment naïve patients, the single-arm phase 2 KEYNOTE-427 trial treated patients with pembrolizumab, noting a median PFS of 7.1 months (95% CI 5.6-11.0) and a median OS not reached (95% CI 31.2 to not reached)7.
Dr. McKay notes that the KEYNOTE-564 trial is a randomized double-blind placebo-controlled phase 3 clinical trial representing the gold-standard design providing the strongest possible evidence of causation. The one year of pembrolizumab therapy for this trial is somewhat arbitrary and inferred from other adjuvant studies. Dr. McKay notes that the non-active placebo control arm is appropriate given the mixed data of the benefit of sunitinib on DFS and the limited utilization of adjuvant sunitinib in clinical practice. Delineating patients by risk was important, given that the primary endpoint was investigator-assessed DFS, defined as the time from randomization to the first documented local recurrence or recurrence of distant kidney cancer. Since increasing T-stage (and N+, and M1 NED) increases the risk of recurrence, this is an important consideration.
The primary endpoint of investigator-assessed DFS is an appropriate endpoint for KEYNOTE-564 according to Dr. McKay. First, it is used for traditional approval for breast cancer, colorectal cancer, GIST, melanoma, and RCC. Second, in 2003, the ODAC consensus was DFS prolongation represented a clinical benefit if the magnitude of benefit outweighed toxicity of adjuvant treatment. Third, per FDA regulatory guidelines, the independent blinded review is not always necessary for DFS assessment, especially in the context of a blinded study design (accurately reflected in real-world practice). Fourth, imaging assessments were performed at the same time points in each treatment arm and continued until disease recurrence, the start of new treatment, death, or withdrawal to limit assessment bias. Fifth, the study did not censor deaths without tumor progression limiting the risk of overestimation of DFS.
Looking at the baseline characteristics of patients in KEYNOTE-564, Dr. McKay notes that the majority of patients were M0 intermediate-high risk (86.1% in the pembrolizumab arm and 86.9% in the placebo arm). Additionally, most patients had a PD-L1 status of CPS >=1 (73.6% in the pembrolizumab arm and 76.9% in the placebo arm). She would like to see a breakdown by type of nephrectomy given that local disease recurrence was included as a DFS event. KEYNOTE-564 was a positive clinical trial with a DFS advantage for pembrolizumab with a 32% reduction in the risk of recurrence or death. Looking closer at the Kaplan-Meier curve, Dr. McKay highlights that we start to see early separation of the curves at the first scan assessment at 12 weeks. Subsequently, during the continued follow-up with additional scan assessments, the curves continue to separate until ~36 months follow-up where the curves cross. However, this tail in the curve needs to be interpreted with caution given the limited patients at risk and need for longer follow-up.
The subgroup analyses of KEYNOTE-564 showed that the hazard ratio point estimate favors pembrolizumab for all subgroups, importantly with benefit independent of PD-L1 status, and with a benefit in the M1 group, albeit with a small number of patients. Dr. McKay notes that additional subgroup analyses by pT, pN, sarcomatoid differentiation and grade are warranted to provide further context into patients that may benefit from treatment. Despite only 51 events (26% of events needed for the final OS analysis), a signal is emerging for a potential OS benefit, but additional follow-up is needed and planned. Furthermore, Dr. McKay suggests that data patterns of progression (local versus distant) and subsequent treatments (surgical/radiation or systemic) are warranted. Toxicity was comparable to that observed in the metastatic setting, unlike the adjuvant experience with VEGF TKIs.
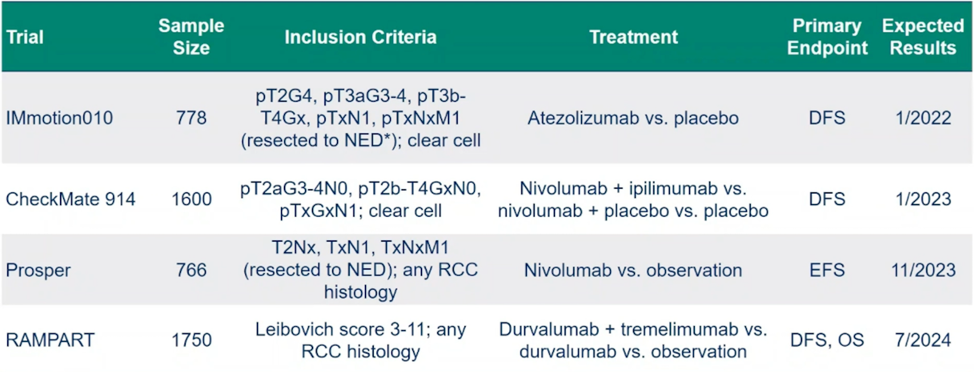
Ultimately, the decision for adjuvant therapy comes down to balancing (i) disease-free survival, (ii) overall survival, (iii) risk of overtreatment, (iv) side effects of therapy, (v) quality of life, and (vi) financial cost. There are several studies of adjuvant checkpoint inhibitor therapy that are ongoing and we will eagerly await their results over the next several years:
Dr. McKay concluded her presentation suggesting that yes, KEYNOTE-564 is practice-changing data:
- It represents a paradigm shift as the first positive phase 3 study of adjuvant immunotherapy in RCC
- DFS prolongation represents clinical benefit given the magnitude of benefit and limited toxicity
- Longer follow-up will be needed to assess the impact on OS
- If adjuvant pembrolizumab is adopted, new questions will arise: Should there be broad implementation for all patients? Is there an application for non-clear cell patients? If recurrence develops, how does this alter first-line treatment for advanced disease?
At the end of the day, Dr. McKay notes that this is a quantum leap for patients into the next phase of options for renal cell carcinoma!
Presented By: Rana R. McKay, MD, University of California San Diego, Moores Cancer Center
Written By: Zachary Klaassen, MD, MSc, Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021
References:
- Haas NB, Manola J, Uzzo RG, et al. Adjuvant sunitinib or sorafenib for high-risk, non-metastatic renal-cell carcinoma (ECOG-ACRIN E2805): A double-blind, placebo-controlled, randomised, phase 3 trial. Lancet 2016;387(10032):2008-2016.
- Ravaud A, Motzer RJ, Pandha HS, et al. Adjuvant Sunitinib in High-Risk Renal-Cell Carcinoma after Nephrectomy. N Engl J Med 2016;375(23):2246-2254.
- Motzer RJ, Haas NB, Donskov F, et al. Randomized phase III trial of adjuvant pazopanib versus placebo after nephrectomy in patients with locally advanced renal cell carcinoma (RCC) (PROTECT). J Clin Oncol 2017;35(35):3916-3923.
- Gross-Goupil M, Kwon TG, Eto M, et al. Axitinib versus placebo as an adjuvant treatment of renal cell carcinoma: results from the phase III, randomized ATLAS trial. Ann Oncol 2018 Dec 1;29(12):2371-2378.
- Eisen T, Frangou E, Oza B, Ritchie AWS, et al. Adjuvant sorafenib for renal cell carcinoma at intermediate or high risk of relapse: Results from the SORCE randomized phase III Intergroup Trial. J Clin Oncol. 2020 Dec 1;38(34):4064-4075.
- Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 2015;373(19):1803-1813.
- McDermott DF, Lee JL, Ziobro M, et al. Open-label, single-arm, phase II study of pembrolizumab monotherapy as first-line therapy in patients with advanced non-clear cell renal cell carcinoma. J Clin Oncol. 2021 Mar 20;39(9):1029-1039.


