While the methods of CheckMate 9ER have been previously presented and published, in brief, patients with advanced clear cell RCC were randomized in a 1:1 fashion to nivolumab 240 mg IV Q2W and cabozantinib 40 mg PO daily or sunitinib 50 mg PO daily (4 weeks of 6-week cycles). The 320 patients in the nivolumab and cabozantinib arm were allowed cabozantinib dose reductions to 20 mg PO daily or 20 mg PO every other day to manage adverse events.
In this analysis, the authors performed time-to-event Cox proportional hazard exposure-response analysis to characterize the relationship between predicted cabozantinib exposure or apparent clearance (CL/F) and specified endpoints, including PFS, dose modification, and select adverse events of interest including palmar-plantar erythrodysesthesia [PPE; Gr ≥1], diarrhea [Gr ≥3], hypertension [Gr ≥3], fatigue/asthenia [Gr ≥3], and ALT/AST elevation [Gr ≥3]. Cabozantinib exposure was defined as the overall average concentration from time zero to the time of event or censoring for each endpoint and estimated by population pharmacokinetic modeling. Due to a low number of events, this analysis was not performed for overall survival.
Assessing progression-free survival, the predicted cabozantinib exposure at 40-mg and 20-mg daily doses was not significantly associated with the rate of progression or death (HR 1.00, 95% CI 0.78–1.27, for 20-mg vs 40-mg dose).
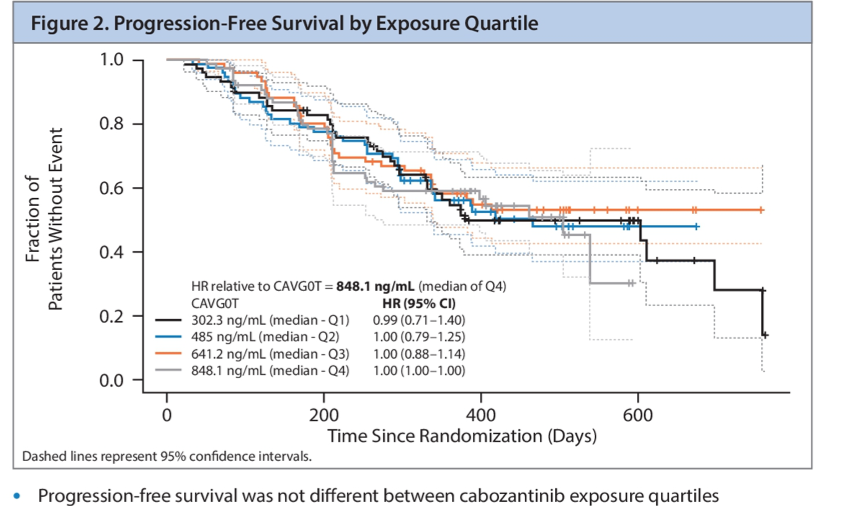
Further, in predicted survival analysis based on constant average cabozantinib concentrations, cabozantinib exposure was not a significant predictor of progression-free survival.
In a similar exposure-response analysis focused on adverse events, lower predicted cabozantinib exposure was significantly associated with lower rates of PPE (HR 0.63, 95% CI 0.50–0.78, for 20-mg vs 40-mg dose) and diarrhea (HR 0.48, 95% CI 0.29–0.80) but was not with rates of hypertension, fatigue/asthenia, or ALT/AST elevation.

Interestingly, while the risk of cabozantinib dose modification increased with decreasing cabozantinib clearance, this effect was not significant.
The authors conclude that, based on exposure-response modeling of patients receiving first line combination therapy with nivolumab and cabozantinib for advanced RCC in the CheckMate 9ER trial, cabozantinib exposure was not significantly associated with PFS, though higher cabozantinib exposure was associated with higher rates of PPE and diarrhea. This modeling therefore supports the notion that dose modifications, as necessary to manage cabozantinib toxicity will not adversely affect the efficacy of this combination therapy.
Presented by: Amishi Y. Shah, MD, Department of Genitourinary Medical Oncology, Division of Cancer Medicine
Written by: Christopher J.D. Wallis, Urologic Oncology Fellow, Vanderbilt University Medical Center, Contact: @WallisCJD on Twitter at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021


