(UroToday.com) Pembrolizumab was approved for cisplatin-ineligible patients with untreated advanced urothelial carcinoma based on initial results of the phase 2 KEYNOTE-052 study, which showed an objective response rate of 24%.1 Updated results after two years of follow-up were consistent, showing an objective response rate of 29%.2 At the 2021 ASCO annual meeting, Dr. Peter O'Donnell and collaborators presented updated results of the KEYNOTE-052 trial after up to 5 years of follow-up.
KEYNOTE-052 is a single-arm, multi-site, open-label trial. Patients had advanced or metastatic urothelial carcinoma, were cisplatin ineligible (criteria: ECOG PS 2, CrCl ≥30 to ̃60 mL/min, grade ≥2 peripheral neuropathy/hearing loss, NYHA class III heart failure), and had not previously received chemotherapy for advanced/metastatic disease. Patients received pembrolizumab 200 mg IV Q3W until progression, unacceptable toxicity, withdrawal, or 24 months of therapy, whichever occurred first. PD-L1 status was determined by combined positive score (CPS, number of PD-L1–staining cells [tumor cells, lymphocytes, macrophages] divided by the total number of viable tumor cells, multiplied by 100); PD-L1–positive was denoted as CPS ≥10. The primary end point was confirmed objective response rate (RECIST v1.1, independent central review). Key secondary end points included duration of response, OS, and safety.
Among 370 enrolled patients, the median age was 74 years of age, 315 (85.1%) had visceral disease, and 43 (11.6%) completed 24 months of therapy. Median time from enrollment to data cutoff (September 26, 2020) was 56.3 months (range, 51.2-65.3) for all patients and 56.0 months (range, 51.4-65.2) for the 110 patients (29.7%) with CPS ≥10. The current patient disposition for this updated analysis is as follows: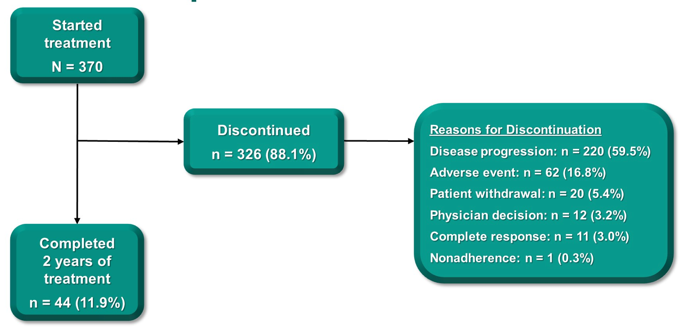
Confirmed objective response rate for all patients was 28.9% (95% CI, 24.3-33.8), with a complete response of 9.5% (n=35), and a partial response rate of 19.5% (n=72). Median duration of response was 33.4 months (range, 1.4+ to 60.7+), including 44.8% and 39.4% of patients with a duration of response ≥36 and ≥48 months, respectively, based on Kaplan-Meier estimates. The median OS was 11.3 months (95% CI, 9.7-13.1):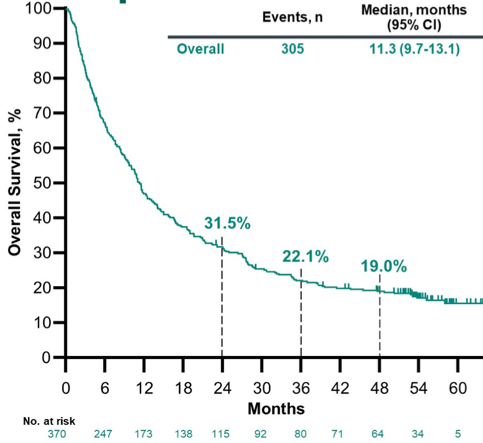
The 24- and 36-month OS rates were 31.5% and 22.1%. Patients with CPS ≥10 had better outcomes than patients with CPS <10: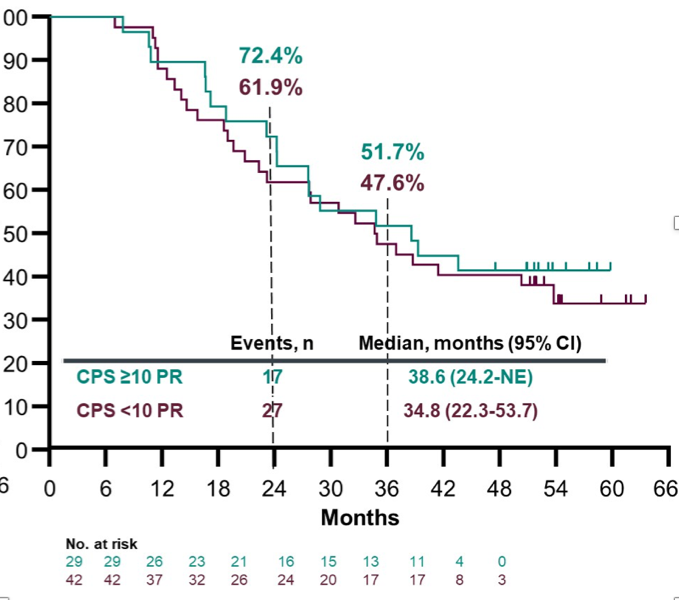
Of the 331 evaluable patients, 193 (58.3%) experienced any reduction in target lesion size, including 136 (41.1%) of patients that had a target lesions reduction of >= 30%, and 90 (27.2%) that had a target lesions reduction of >= 60%. Treatment-related adverse events occurred in 67.3% of patients, with 21.1% of treatment-related adverse events being grade ≥3, including 1 death (myositis).
Dr. O’Donnell concluded his updated analysis of KEYNOTE-052 with the following summary points:
- First-line pembrolizumab monotherapy continued to show durable antitumor activity up to 5 years after the last patient was enrolled, with an objective response rate of 28.9%, median duration of response of 33.4 months, and median OS of 11.3 months
- Patients with CPS ≥10 were more likely to respond than those with CPS <10, and this response was durable, supporting the current FDA indication
- Safety was consistent with the known profile of pembrolizumab
- These data support the use of pembrolizumab in cisplatin-ineligible patients with locally advanced or metastatic urothelial carcinoma
Clinical trial information: NCT02335424
Presented by: Peter H. O'Donnell, MD, Associate Professor of Medicine, The University of Chicago, Chicago, IL
Co-Authors: Arjun Vasant Balar, Jacqueline Vuky, Daniel Castellano, Joaquim Bellmunt, Thomas Powles, Dean F. Bajorin, Petros Grivas, Noah M. Hahn, Elizabeth R. Plimack, Jin Zhi Xu, James Luke Godwin, Blanca Homet Moreno, Ronald De Wit; Perlmutter Cancer Center, NYU Langone Health, New York, NY; Oregon Health and Science University, Portland, OR; Hospital Universitario 12 de Octubre, Madrid, Spain; Beth Israel Deaconess Medical Center, Harvard Medical School, Boston, MA; Barts Cancer Institute, Queen Mary University of London, London, United Kingdom; Memorial Sloan Kettering Cancer Center, New York, NY; University of Washington, Seattle, WA; The Sidney Kimmel Comprehensive Cancer Center, Johns Hopkins Medicine, Baltimore, MD; Fox Chase Cancer Center, Philadelphia, PA; Merck & Co., Inc., Kenilworth, NJ; Erasmus MC Cancer Institute, Rotterdam, Netherlands
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting #ASCO21, June, 4-8, 2021
References:
- Balar AV, Castellano D, O’Donnell PH, et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): A multicentre, single-arm, phase 2 study. Lancet Oncol 2017;18(11):1483-1492.
- Vuky J, Balar AV, Castellano D, et al. Long-term outcomes in KEYNOTE-052: Phase II Study investigating first-line pembrolizumab in cisplatin-ineligible patients with locally advanced or metastatic urothelial cancer. J Clin Oncol. 2020 Aug 10;38(23):2658-2666.


