- Lack of prospective studies exclusively testing cisplatin-based neoadjuvant chemotherapy
- Lack of rigorous methods to define clinical complete response and its association with long term outcomes
- Limited understanding of the role of “salvage” cystectomy
As such, Dr. Galsky and colleagues posed the question “Can cisplatin-based chemotherapy plus PD-1 blockade refine a TURBT plus systemic therapy approach?” based on the following paradigm:

At the 2021 America Society of Clinical Oncology (ASCO) annual program’s oral abstraction session, Dr. Galsky and colleagues presented results of their phase 2 trial HCRN GU 16-257 assessing gemcitabine, cisplatin, plus nivolumab with selective bladder sparing in patients with muscle-invasive bladder cancer.
Eligible patients were cisplatin-eligible with cT2-T4aN0M0 urothelial bladder cancer. Patients received 4 cycles of gemcitabine, cisplatin, plus nivolumab followed by clinical restaging including urine cytology, MRI/CT of the bladder, cystoscopy and bladder/prostatic urethral biopsies. Patients achieving a clinical complete response (normal cytology, imaging, and cT0/Ta) were eligible to proceed without cystectomy and receive nivolumab q2 weeks x 8 followed by surveillance; otherwise, patients underwent cystectomy. The trial schema for HCRN GU 16-257 is as follows:

Coprimary endpoints included (1) clinical complete response rate and (2) ability of clinical complete response to predict 2-year metastasis-free survival (MFS). Clinical complete response was defined as:
- No abnormalities on post-cycle #4 imaging
- No abnormalities on post-cycle #4 urine cytology
- <= low grade Ta on post-cycle #4 bladder biopsies
The key secondary endpoint was the impact of genomic alterations in baseline TURBT (TMB, ERCC2, FANCC, RB1, ATM) on performance of clinical complete response for predicting MFS. The sample size was generated such that the lower bound of the 95% confidence interval for the positive predictive value for benefit predicted by clinical complete response exceeded 80%. The clinical complete response rate coprimary endpoint, and interim analysis of 1-year outcomes, were reported by Dr. Galsky.
Between August 2018 and November 2020, 76 patients were enrolled at 7 sites (male 79%, median age 69; cT2 = 57%, cT3 = 32%, cT4 = 12%) and 64 (84%) patients have completed post-cycle 4 restaging. Thirty-one of 64 patients achieved a clinical complete response (48%; 95% CI 36%-61%), over a median follow-up for clinical complete response patients of 13.7 months (range: 2.5-24 months). One clinical complete response patient opted for immediate cystectomy (pTaN0M0). The current disposition of the patients in HCRN GU 16-257 is as follows:

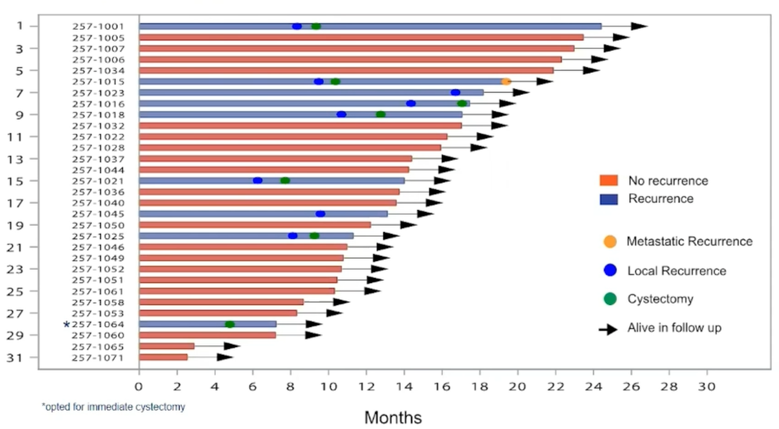
Local recurrence has occurred in 8/31 clinical complete response patients and 6 underwent cystectomy (pT0N0 = 1, pTaN0 = 1, pTisN0 =1, pT2N0 = 2, pT4N1 = 1). The outcomes of patients with complete clinical response are summarized in the following swimmer’s plot:
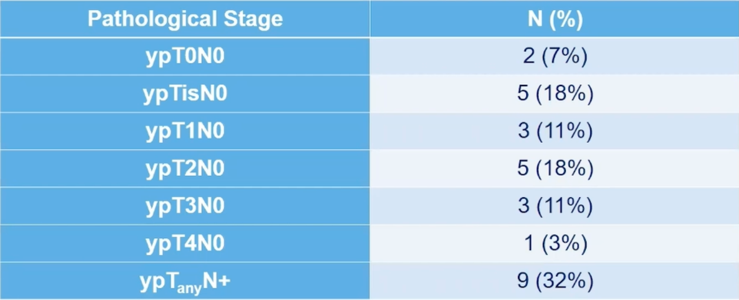
Pathological stage in patients without clinical complete response undergoing immediate cystectomy (n=28) is as follows:
Stopping rules were employed for (i) high-rate of immune-related grade >= 3 adverse events, (ii) high-rate of muscle-invasive and/or metastatic recurrence rates (patients achieving a complete clinical response). Currently, no criteria for early stopping have been met, and the adverse event profile is consistent with other studies of gemcitabine + PD-1 blockade.
Among 63 patients for which genomic alterations and clinical complete response could be assessed, TMB ≥ 10 mut/Mb (p=0.02) or mutant ERCC2 (p=0.02) were associated with clinical complete response or partial complete response. However, ATM, FANCC, or RB1 alterations were not associated with complete clinical response or partial clinical response. Correlation of genomic alterations with more relevant endpoints (ie. bladder intact long-term survival) requires longer follow-up of these patients.
Dr. Galsky concluded his presentation of the HCRN GU 16-257 trial with the following take-home messages:
- TURBT plus gemcitabine, cisplatin, plus nivolumab achieves stringently defined clinical complete response in a large subset of patients with muscle-invasive bladder cancer
- 1-year bladder intact survival is possible though the durability of responses requires longer follow-up
- The adverse event profile is consistent with other reports of gemcitabine + PD-1/PD-L1 blockade
- A suite of genomic and radiomic biomarkers are being explored in an effort to refine the ability of clinical complete response to predict long-term bladder-intact recurrence-free survival
Clinical trial information: NCT03558087
Presented by: Matt D. Galsky, MD, Division of Hematology and Medical Oncology, Tisch Cancer Institute, Icahn School of Medicine at Mount Sinai, New York, NY
Co-Authors: Siamak Daneshmand, Kevin G. Chan, Tanya B. Dorff, Jeremy Paul Cetnar, Brock O Neil, Anishka D'souza, Ronac Mamtani, Christos Kyriakopoulos, Philip Garcia, Sudeh Izadmehr, Menggang Yu, Qianqian Zhao, Reza Mehrazin, Sara C Lewis, John Sfakianos, Sumanta K. Pal; USC Norris Comprehensive Cancer Center, Los Angeles, CA; City of Hope, Duarte, CA; Oregon Health and Science University, Portland, OR; Huntsman Cancer Institute, University of Utah, Salt Lake City, UT; Division of Oncology, USC Keck School of Medicine, Norris Comprehensive Cancer Center, Los Angeles, CA; University of Pennsylvania Abramson Cancer Center, Philadelphia, PA; University of Wisconsin Carbone Cancer Center, Madison, WI; Tisch Cancer Institute, Division of Hematology/Medical Oncology, Icahn School of Medicine at Mount Sinai, New York, NY; Icahn School of Medicine at Mount Sinai, New York; University of Wisconsin Department of Biostatistics and Medical Informatics, Madison, WI; University of Wisconsin Madison, Madison, WI; Icahn School of Medicine at Mount Sinai, New York, NY; Department of Medical Oncology & Therapeutics, City of Hope Comprehensive Cancer Center, Duarte, CA
Written by: Zachary Klaassen, MD, MSc – Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia Twitter: @zklaassen_md at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021

