This is a phase Ib multi-institutional trial in patients with localized muscle-invasive bladder cancer treated with two neoadjuvant doses (4 weeks apart) of nivolumab alone (480 mg) in cohort 1 or nivolumab (480 mg) + lirilumab (240 mg) in cohort 2 prior to radical cystectomy without adjuvant therapy. Cohorts were enrolled sequentially and were not randomized. Key eligibility criteria included stage cT2-4aN0-1M0, ≥20% tumor content at TURBT, and cisplatin-ineligibility (Galsky criteria) or refusal. The primary endpoint was safety manifested as the rate of ≥ grade 3 treatment-related adverse events assessed in each cohort with CTCAE v5.0. Key secondary endpoints included the percentage of patients who had radical cystectomy > 6 weeks after last neoadjuvant dose due to treatment-related adverse events, CD8+ T cell density at radical cystectomy, ypT0N0 and < ypT2N0 rates, CD8+ T cell density change between TURBT and radical cystectomy, recurrence-free survival (RFS) and biomarkers in tumor tissue, blood, and urine. The trial design for PrE0907 is as follows:
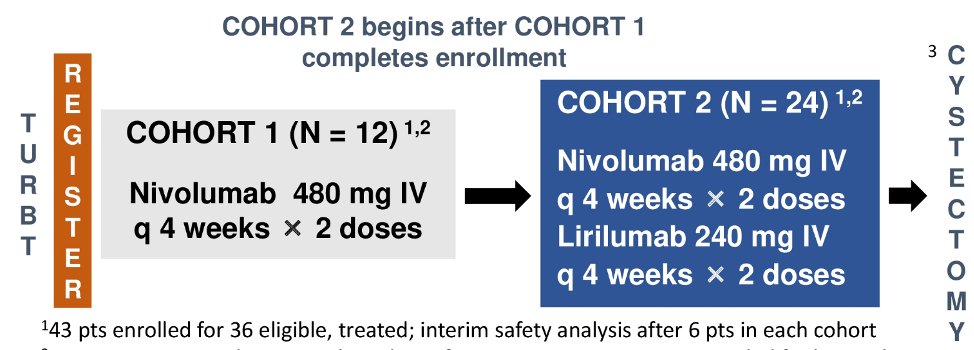
Among 43 patients enrolled (13 cohort 1, 30 cohort 2), the median age was 75 (51-89), 67% were men, all had performance status ECOG 0-1. Patients were cisplatin-ineligible due to impaired renal function (47%) and hearing loss (37%), while 14 % refused cisplatin. At baseline, 37 patients had cT2 stage, two had cN1, and three cNx disease. In cohort 1 and 2, 13 and 29 patients, respectively, completed intended neoadjuvant treatment, and 41/43 underwent radical cystectomy (12/13 cohort 1, 29/30 cohort 2). One patient progressed to metastatic disease prior to radical cystectomy (cohort 1) and one withdrew consent prior to being treated (cohort 2). Additionally, 1 patient was found to have cervical cancer at radical cystectomy. The median time from the last neoadjuvant dose to radical cystectomy was 27 (95% CI 24-29) days. There was no radical cystectomy delayed > 6 weeks from treatment completion due to treatment-related adverse events. Grade 3 treatment-related adverse events occurred in 0% with nivolumab and 6.7% (90%CI 1.2-19.5%) in nivolumab + lirilumab (1: arthralgia, 1: gout, 2: hip pain) that all resolved, and there were no Grade 4/5 treatment-related adverse events occurred. Of 40 patients with muscle-invasive bladder cancer and radical cystectomy, ypT0N0 rates for nivolumab and nivolumab + lirilumab were 8% and 18%, while < ypT2N0 rates were 17% and 29%, respectively. Data on RFS and OS, and biomarker data were not yet mature.
Dr. Grivas concluded his presentation of the PrE0807 trial with the following summary points:
- Neoadjuvant nivolumab alone and nivolumab + lirilumab combination prior to radical cystectomy were safe, feasible, and well-tolerated in cisplatin-ineligible patients with muscle-invasive disease
- ypT0N0 rates were unexpectedly low, especially with nivolumab alone
- The 2-year RFS as well as blood, urine, and tissue-based marker assessment
- Two phase 3 trials (NCT03661320; NCT04209114) are evaluating the peri-operative role of nivolumab + chemotherapy +/- Linrodostat in cisplatin-fit and nivolumab +/- Bempeg in cisplatin unfit patients and are also assessing biomarkers
Clinical trial information: NCT03532451
Presented By: Petros Grivas, MD, Ph.D., University of Washington, Fred Hutchinson Cancer Research Center, Seattle Cancer Care Alliance, Seattle, WA
Written By: Zachary Klaassen, MD, MSc, Urologic Oncologist, Assistant Professor of Urology, Georgia Cancer Center, Augusta University/Medical College of Georgia, Twitter: @zklaassen_md at the 2021 American Society of Clinical Oncology (ASCO) Annual Meeting, Virtual Annual Meeting #ASCO21, June, 4-8, 2021


