Arlene O. Siefker-Radtke, MD, presented the first interim analysis of FIERCE-22. FIERCE-22 is a single arm phase Ib/II trial of the FGFR3 antibody vofatamab (with a two week lead-in period) in combination with the anti-PD-1 checkpoint inhibitor pembrolizumab in patients with platinum-refractory metastatic urothelial cancer. Results are stratified by FGFR3 mutational status.
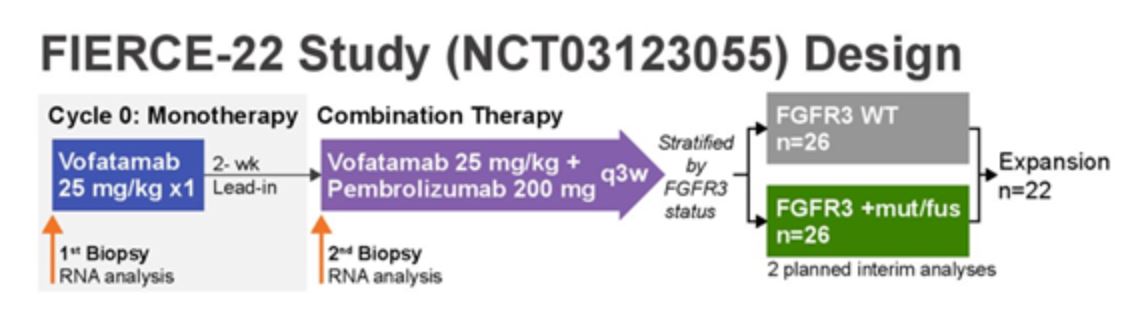
With 28 patients enrolled thus far, 22 were evaluable for response, among whom investigators report an overall response rate of 32% (7/22, all PR). Interestingly, while the response to vofatamab did not seem to correlate with FGFR3 mutation or fusion, molecular subtyping revealed that the luminal subtype – associated with immunologically “cold” tumors – seems to enrich for responses. Furthermore, the post-vofatamab lead-in tissue biopsies reveal upregulation of key inflammatory genes, suggestive of an immunologic mechanism of action.

Combination vofatamab with pembrolizumab was well-tolerated, with only five patients discontinuing for toxicity, and no dose reductions occurred. The most common grade ≥3 adverse events were anemia (11%), urinary tract infection (8%) and acute kidney injury (6%). Of note, while hyperphosphatemia and ocular toxicity are associated with erdafitinib, these toxicities were not encountered with vofatamab.

Further study is needed to better understand the synergy between FGFR inhibition and checkpoint blockade immunotherapy. Matured safety and efficacy data are eagerly anticipated.
Presented by: Arlene O. Siefker-Radtke, MD, Professor, Department of Genitourinary Medical Oncology, Division of Cancer Medicine, The University of Texas MD Anderson Cancer Center, Houston, TX
Written by: Michael Lattanzi, MD, Internal Medicine Resident, Department of Medicine, NYU School of Medicine, @MikeLattanzi at the 2019 ASCO Annual Meeting #ASCO19, May 31- June 4, 2019, Chicago, IL USA
References:
- Rosenberg JE, Hoffman-Censits J, Powles T, Van Der Heijden MS, Balar AV, Necchi A, Dawson N, O'Donnell PH, Balmanoukian A, Loriot Y, Srinivas S. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. The Lancet. 2016 May 7;387(10031):1909-20.
- Siefker-Radtke AO, Necchi A, Park SH, GarcÃa-Donas J, Huddart RA, Burgess EF, Fleming MT, Rezazadeh A, Mellado B, Varlamov S, Joshi M. First results from the primary analysis population of the phase 2 study of erdafitinib (ERDA; JNJ-42756493) in patients (pts) with metastatic or unresectable urothelial carcinoma (mUC) and FGFR alterations (FGFRalt).


